Advances in Animal and Veterinary Sciences
Case Report
Oral Papillomatosis in a Dog: Surgical Management and Histopathological Findings
Khan Sharun1*, Kalaiselvan E1, Sindhoora K2, Faslu Rahman AT2, Azam Khan1, AM Pawde1, Amarpal1
1Division of Surgery, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India; 2Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India.
Abstract | Canine oral papillomatosis is a viral disease that commonly affects young dogs. A six-month-old male German shepherd dog was presented with a history of progressively developed nodular growth in the lip, gingiva and tongue. All physical parameters were found to be normal. The animal was pre-medicated using atropine, butorphanol and diazepam as per the standard pre-anesthetic protocol. General anaesthesia was induced using ketamine hydrochloride. General maintenance was provided using Ketamine-diazepam mixture and the nodular masses were removed using a combination of surgical excision and electrocautery. Post-operatively, the animal was treated with antibiotics for five days and anti-inflammatory drugs for three days. Excised tissue samples were processed and subjected to standard histopathological examination. Histopathologic examination revealed a diffuse epidermal hyperplasia, marked neovascularization, koilocytes with clear perinuclear vacuolization and keratinocytes with keratohyalin granules. The findings were suggestive of canine oral papilloma virus induced papillomatosis. The animal made an uneventful recovery without any recurrence. This paper describes the successful surgical management of canine oral papillomatosis without any recurrence and its peculiar histopathological findings.
Keywords | Canine, Papillomatosis, Wart, Electrocautery, Surgical excision
Received | November 12, 2019; Accepted | March 12, 2020; Published | March 25, 2020
*Correspondence | Khan Sharun, Division of Surgery, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India; Email: [email protected]
Citation | Sharun K, Kalaiselvan E, Sindhoora K, Faslu Rahman AT, Azam Khan, AM Pawde, Amarpal (2020). Oral papillomatosis in a dog: Surgical management and histopathological findings. Adv. Anim. Vet. Sci. 8(4): 408-411.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.408.411
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sharun et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Canine oral papillomatosis is a viral disease of dogs, which is caused by the canine oral papillomavirus (CPV) that comes under the family Papovaviridae (Nicholls and Stanley, 1999). Canine oral papillomavirus consists of a group having eight viruses, which are represented by CPV-1 to CPV-8. Among these viruses, CPV-1 is responsible for causing canine oral papillomatosis in young dogs (Lange and Favrot, 2011). The Papillomavirus is a DNA virus that has special affinity towards the cutaneous squamous or mucosal epithelium (Gross et al., 2008). Canine papillomatosis is commonly seen in mixed-breed dogs of age in between 6 months to 10 years, and majority of the lesions are seen in skin followed by lips (Bianchi et al., 2012). Studies conducted by Sundberg et al., 1994 have reported that dogs with immunosuppressive disorders are highly predisposed to canine papillomatosis. Canine oral papillomatosis is characterized by the presence of multiple cauliflower-like masses seen commonly on the tongue, lips, palate gingiva, buccal mucosa and pharynx (Teifke et al., 1998).
Canine oral papillomatosis has a specialized ability for spontaneous regression due to development of cell-mediated immune responses that may develop over a period of up to 12 months in naturally infected animals (Sancak et al., 2015). Hence surgical management is indicated in most cases for the immediate removal of papillomas due to its superior results when compared to other modes of therapy. Canine tumors can be treated using therapeutic strategies involving different modes of treatments such as surgical excision, chemotherapy, radiation therapy, and immunotherapy (Sharun et al., 2019)
The present paper reports a case of canine oral papillomatosis involving lips, gingiva and tongue that was managed by surgical excision and electrocautery.
Case history and clinical examination
A six month old male German shepherd dog was presented with a history of nodular growth in the lip, gingiva and tongue that developed progressively over a period of one month. The owner reported that the animal had progressive loss of appetite during this period. On physical examination, the animal showed normal rectal temperature (102.5 °F), respiratory rate (20/minute), and heart rate (90 bpm). Clinical examination revealed presence of multiple nodular growth (>25) of 2-16 mm diameter, which was dispersed over the oral mucocutaneous junction involving the lips and the gingiva, which was visible externally. Examination of the oral cavity further identified multiple (>12) hard pale pink cauliflower shaped nodules of 3-15 mm diameter in the tongue margin (Figure 1). Due to the extensive nature of the nodular mass, it was decided to surgically excise it using electrocautery under general anaesthesia.

Figure 1: (a) Gross appearance of nodular masses having different sizes present in the lips and gingiva. (b) Multiple nodular mass present at the tongue margin.
Surgical management
The surgical site was prepared aseptically and diluted povidone iodine (1% w/v) was used to flush the oral cavity prior to the surgery. Preoperative antibiotic therapy was initiated using intravenous ceftriaxone at the dose rate of 25mg/kg bodyweight. The patient was premedicated using atropine sulfate at the dose rate of 0.04mg/kg body weight intramuscularly. After 10 minutes, butorphanol was given at the dose rate of 0.2 mg/kg body weight intravenously which was followed by diazepam at the dose rate of 0.5 mg/kg body weight intravenously. Induction of general anaesthesia was achieved by using Ketamine at the dose rate of 10 mg/ kg body weight. A cuffed endotracheal tube was placed into the trachea to provide oxygen at the flow rate of 1.5 L/minute. The anesthesia was maintained using diazepam-ketamine mixture at a ratio of 1:1 to effect.
The nodular masses, present in the lips, gingiva and tongue, were excised one after another carefully. Electrocautery was used to control the bleeding and vicryl 2-0 was used to ligate bigger bleeding points in cases where electrocautery failed to control the bleeding. All of the nodular masses were removed completely by surgical excisions (Figure 2). The defects, produced secondary to excised nodular mass, were sutured using vicryl (2-0). Post-operatively, animal was treated with ceftriaxone at the dose rate of 25mg/kg bodyweight intravenously for five days along with meloxicam at the dose rate of 0.2 mg/kg body weight intramuscularly for three days. During the initial 48 hours, enteric nutrition was withdrawn and the animal was provided with parenteral fluid therapy to facilitate healing. The owner was advised to apply chlorhexidine gluconate gel (1% w/w) at the surgical site. The surgical wound healed within 7 days of performing surgery. The animal made an uneventful recovery without any recurrence during the surveillance period of one year.
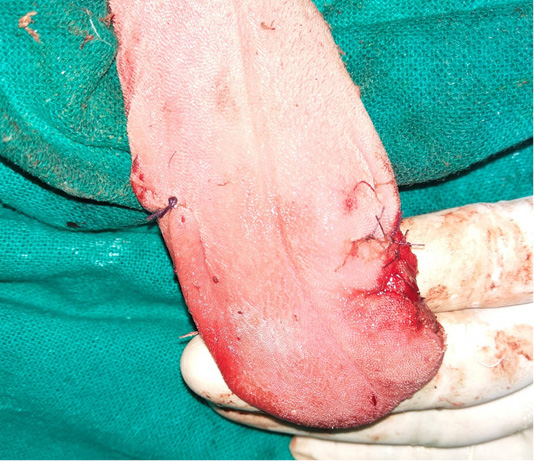
Figure 2: Reconstructed tongue margins following the surgical excision of nodular cauliflower shaped nodules.
Histopathology
The excised nodular mass was preserved in 10% buffered formalin which was later processed in routine manner. Following processing and sectioning, the tissue sample was stained using Haematoxylin and Eosin (H and E).
Histopathological examination of stained tissue sections revealed diffused epidermal hyperplasia with parakeratosis and broad elongated rete pegs (epithelial extensions that project into the underlying connective tissue). There was a marked neovascularization in superficial dermis underlying the acanthotic epidermis. In stratum granulosum and stratum spinosum, few numbers of koilocytes with clear perinuclear vacuolization were noticed and many keratinocytes contain clumps of giant keratohyalin granules. These characteristic histopathological findings indicate that this is a case of papillomatosis suggestive of viral etiology (Figures 3 and 4). Based on gross appearance and histopathological examination, it was diagnosed as a case of canine oral papillomatosis.

Figure 3: Histomicrograph showing diffuse epidermal hyperplasia with parakeratosis (arrows), broad and elongated rete pegs (arrowhead) and neovascularization of superficial dermis (star). H and E, 10x.
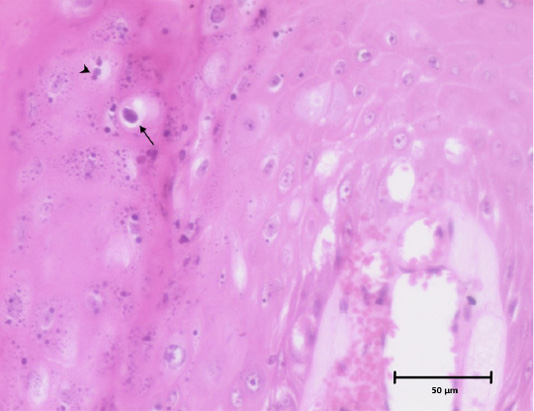
Figure 4: Histomicrograph showing koilocyte with a clear perinuclear cytoplasmic vacuolization (arrow) and clumps of keratohyalin granules (arrow head) in stratum granulosum of epidermis. H and E, 10x.
DISCUSSION
Canine oral papillomatosis is generally diagnosed based on the histopathological findings. Microscopic examination of stained section is characterized by presence of prominent epithelial proliferations with thickened epidermis. The keratinocytes present in the stratum granulosum or spinosum had prominent nucleus (Yhee et al., 2010). Canine papillomatosis can also be effectively managed medically using different drugs. Azithromycin is considered as a safe and effective therapeutic agent for the management of papillomatosis in canines. It is used at a dose rate of 10 mg/kg every 24 hour for a period of 10 days for the management of oral and cutaneous canine papillomatosis (Yaǧcı et al., 2008). Recombinant feline interferon-ω is a good choice for the management of canine papillomatosis cases, which are not responsive to other modes of therapy (Fantini et al., 2015). Surgical excision is one of the immediate technique that is used for managing canine oral papillomatosis. But there is a possibility of recurrence. In cases which are refractory to surgical excision, treatment with a recombinant canine oral papillomavirus vaccine gives good result. Humoral immune response is produced when canine oral papillomavirus major coat protein L1 is systemically administered (Kuntsi-Vaattovaara et al., 2003). Oral administration of the antiviral drug Acyclovir is another option in managing canine oral papillomatosis. A ten day treatment with oral Acyclovir was found to be effective in managing papillomatosis resulting in complete remission (Uwagie-Ero et al., 2017). But further studies should be conducted in a larger population to evaluate its efficacy.
Canine transmissible venereal tumor (CTVT) is another tumor that produces friable, fleshy, and cauliflower like mass in the oral cavity, and this tumor can be identified histopathologically. CTVT responds well to the treatment with chemotherapeutic agent known as vincristine (Rezaei et al., 2016). Even though there are many well-established treatment protocols for managing canine oral papillomatosis, it is difficult to evaluate the efficacy of different therapy due to the self-limiting nature of the disease. This might be the reason why each case of canine papillomatosis responds unpredictably to different modes of treatment. In the present case, the dog had multiple oral papillomas spread over lips, gingiva, and the tongue, which were managed effectively by surgical excision alone with the help of electrocautery. Post-operatively, no recurrences were reported in the present case that indicated the effectiveness of surgical excision in managing canine oral papillomatosis. The histological findings were conclusive in diagnosing the case to be canine oral papilloma virus-induced papillomatosis.
CONCLUSIONS
Histopathological examination revealed diffuse epidermal hyperplasia, marked neovascularization, and koilocytes with clear perinuclear vacuolization and keratinocytes with keratohyalin granules suggestive of papillomatosis. These characteristic histopathological findings were suggestive of canine oral papillomatosis due to viral etiology. Selection of a single but superior method of managing oral papillomatosis will be difficult. But surgical excision can be always considered as the bare minimum requirement in managing non-metastatic but contagious tumors like canine oral papillomatosis due to its immediate results. Other therapeutic agents can be used as an add-on to the surgical excision for obtaining superior results either for preventing the recurrence or in cases of incomplete surgical excision.
AUTHORS CONTRIBUTION
KS collected clinical data, drafted and revised the manuscript. KE, SK, FRAT, AK interpreted the clinical and histopathological data. AMP and A critically revised the manuscript.
Ethical approval
This article does not contain any studies with human or animal participants performed by any of the authors. All protocols followed were as per the guidelines from the standard textbooks in Veterinary Medicine and Surgery and were ethical.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES






