Advances in Animal and Veterinary Sciences
Research Article
Effect of Delayed Serum Separation on Glucose Concentration of Dogs at Room Temperature
Bishal Chand1*, Deepak Subedi1, S.K. Poudel2
1Paklihawa Campus, Institute of Agriculture and Animal Science, Tribhuvan University, Nepal; 2Animal Medical Center, Kathmandu, Nepal.
Abstract | Blood glucose determination is one of the most common clinical diagnostic tests. Delays between blood sample collection and analysis are common in veterinary medicine in field conditions. Since, blood cells continuously metabolize glucose via anaerobic glycolysis, the time of determination of blood glucose after drawing the blood is important. Observational study was carried out to study the change in glucose concentration due to delay in serum separation from January 2019 to March 2019. A total of 35 blood samples (10ml from each dog) were taken from apparently healthy dogs. Whole blood specimens were divided into 14 clot activator tubes and one tube was allowed to clot for 20 mins and then centrifuged immediately. Thus, obtained glucose value was considered as baseline value. The remaining samples were stored at 25°C, and serum glucose value was measured by serum analyzer at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 24 hours post collection. Glucose concentrations were compared using Repeated Measures ANOVA. The decline in serum glucose concentration for all samples stored at 25°C was found to be statistically significant (p<0.05). Mean glucose value of 1 hour was statistically different (p<0.05) from baseline value. At 25°C, serum glucose concentration was found to be decreasing with mean of 10.8% per hour. Hence, blood glucose should be determined within as short a time as possible after drawing the blood from dogs.
Keywords | Blood, Glucose, Glycolysis, Room temperature, Storage
Received | May 11, 2020; Accepted | June 28, 2020; Published | July 15, 2020
*Correspondence | Bishal Chand, Paklihawa Campus, Institute of Agriculture and Animal Science, Tribhuvan University, Nepal; Email: [email protected]
Citation | Chand B, Subedi D, Poudel SK (2020). Effect of delayed serum separation on glucose concentration of dogs at room temperature. Adv. Anim. Vet. Sci. 8(8): 800-803.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.8.800.803
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Chand et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Most energy for cellular activities is derived from glucose, and more than 70% of the energy used by the body is provided by the glucose oxidation process, which is important in maintaining the body’s normal physiological functions (Zhu et al., 2017). Normally in mammals, the body maintains the blood glucose level at a reference range between 70 and 100 mg/dl before-meal (Baker et al., 1969). Glucose can be measured in whole blood, serum or plasma (Tauk et al., 2015). Under usual circumstances, the concentration of glucose in whole blood is about 15% lower than in plasma or serum, but the difference will be less in patients with low hematocrits (McMillin, 1990). Glucose is the primary substrate for the energy needs of RBCs from all species except for the pig (Suzuki et al., 1984). Serum or plasma samples not promptly separated from RBC may contain artifactually low glucose due to the continued uptake and metabolism of glucose by RBC in vitro (O’Neill, 2000). In almost all mammalian species studied, erythrocytes fulfill their energy requirements via consumption of glucose through anaerobic glycolysis, which is also known as the Embden–Meyerhof pathway (EMP) (Collicutt et al., 2015). Glycolysis may consume 5%–7% of the sample’s glucose content per hour in case of human samples (Mikesh and Bruns, 2008). At 22 °C, glucose concentration decreases approximately 10% every 30 to 60 minutes, and this may occur more rapidly if large concentrations of metabolically active cells (e.g., leukocytosis, leukemia) are present (Nelson, 2012). (Thoresen et al., 1992) found that in dog’s sample glucose decreases by 8 to 10 % per hour at 20 ˚C. It is generally recommended that glucose estimations in serum and plasma samples be carried out immediately upon sample collection, and if not immediately possible, that the specimens should be kept frozen until when the determinations are possible, but certainly within 24 hours (Marjani, 2008). In Veterinary Profession it is difficult or nearly impossible to check the blood glucose level immediately after blood collection especially during field conditions. A large number of specimens are dispatched from peripheral centers to a centralized laboratory over long distances where a delay of 12– 24 h or more occurred and at weekends, this interval may exceed 36 h due to closure of the laboratory (Wu et al., 2017). This may lead to misdiagnose many underlying conditions mainly Diabetes, Hypoglycemia and Adrenal gland dysfunction.
In the developing countries like Nepal due to inconsistent power supply, particularly in the rural areas, immediate sample separation and analysis may not be practicable. This study investigates the time-related changes in glucose concentration of serum. This study also investigates the optimum time up to which glucose remains stable in blood at room temperature and will offer veterinarians clinically-useful, information regarding blood specimen collection and handling, which will prevent false diagnosis of hypoglycemia, or normoglycemia in hyperglycemic patients.
Materials and Methods
This study was conducted in Animal Medical Centre (AMC), Chuchhepati, Kathmandu, Nepal. The study was conducted from January 2019 to March 2019. Thirty-Five healthy adult dogs (8 were client owned and remaining were street dogs) of various body weights weighing from 10kg to 32 kg of either sexes (13 males and 22 females) were used as study population. All the samples were taken from the dog of Kathmandu valley. Sample from client owned dog was taken at Animal Medical Center (AMC) and for street dogs we visited different animal rescue shelters i.e. Kathmandu Animal Treatment Centre, Community Dog Welfare and Shree Animal Rescue. All the dogs were restrained by hand and blood was collected either from jugular vein or cephalic vein puncture. About 10 ml of blood sample was collected from each dog. The collected blood was aliquoted to 14 different serum separating gel tubes (SST). All the samples were stored at room temperature (25˚C). One tube was centrifuged after 20 min post collection at 3000 rpm for 5 minute (Salhen et al., 2018). The glucose value was measured by Erba Chem 5 v3 serum analyzer which is based on glucose oxidase method. The value obtained from the first tube was considered as baseline value (0 hour value). The remaining samples were centrifuged at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 24 hours post collection respectively. Statistical Analysis was done using SPSSv21. Glucose value was quoted as Mean±Standard Error Mean (SEM). Repeated measure ANOVA was used to find the effect of delayed serum separation on glucose concentration. Post hoc analysis was done by TUKEY test. The difference between observations was considered significant at p≤0.05. Verbal approval was taken for blood collection from dogs’ owner after explaining about the research.
Results and Discussion
Table 1 shows the mean glucose value of all samples at different periods along with percentage change in glucose value at each hour (i.e. from 0 hour to 1 hour, 1 hour to 2 hour and so on) and from baseline value to subsequent time points (i.e. from 0 hour to 1 hour, 0 hour to 2 hour. 0 hour to 3 hour and so on). From Table 1 we can state that the rate of glucose decline is in between 10 to 13% per hour averaging 10.8% which is in agreement with (Collicutt et al., 2015). This is also in agreement with (Nelson, 2012) where he concluded that rate of glucose decline is approximately 10% hour at 22°C. It can also be stated from above table that glucose decreases at the rate of 6.8mg/dl/hr. This result is in tally with (Nelson, 2012) where he stated that glucose concentration decreases at the rate of approximately 0.4 mmol/L per hour (7 mg/dL per hour). A box plot showing glucose value at different time is shown in Figure 1. First box plot shows mean glucose value of all the samples at 0-hour, second box plot shows mean glucose value at 1 hour and so on. Here, 13 on X-axis indicate the glucose value at 24 hour post collection.
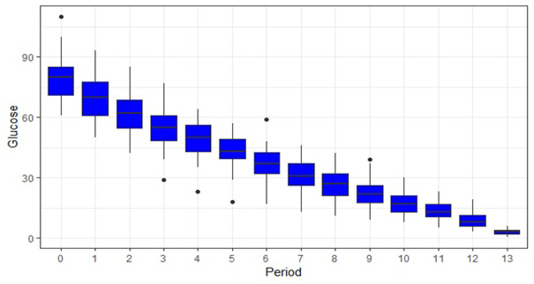
Figure 1: Box plot showing glucose value (mg/dl in Y axis) at different time period(X-axis). Here 13 and 0 on X-axis indicate the mean glucose value of all the samples at 24 hour and baseline glucose value respectively. Mean glucose value at 1 hour is significantly lower (p<0.005) than baseline value and glucose value at 2 hours is significantly lower than value 1 hour.
Statistically significant {F (13,442)= 827.90, p< 0.05} value was found between delay in serum separation and serum glucose concentration. Thus, indicating that glucose decreases significantly as there is delay in serum separation. Table 2 shows p value between each time interval. From this table we can state that there is significant decrease in glucose concentration between each time interval. Lacerda et. (2015) also stated the same finding where they stated that glucose value at 1 hour as different from glucose value from 0 hr. Thus, implying that even 1-hour delay in glucose estimation may cause significant decrease in glucose value at room temperature.
Table 1: Mean glucose value {Mean ± SEM (Standard Error Mean}, percentage change in glucose value (at each hour and from baseline value.
| Time (hours) | Mean glucose value(mg/dl) | Percentage change in glucose value (At each hour) | Percentage change in glucose value (from baseline) |
| (Baseline Value) 0 | 78.83±1.82 | 0 | 0 |
| 1 | 69.80±1.69 | 11.45% | 11.45% |
| 2 | 61.68±1.69 | 11.6% | 21.71% |
| 3 | 55.20±1.72 | 10.5% | 29.97% |
| 4 | 49.2±1.57 | 10.86% | 37.58% |
| 5 | 42.8±1.33 | 12.8% | 45.75% |
| 6 | 36.97±1.28 | 12.6% | 53.1% |
| 7 | 32.77±1.23 | 11.36% | 58.8% |
| 8 | 28.77±1.18 | 12.2% | 63.33% |
| 9 | 25.31±1.15 | 12.02% | 67.89% |
| 10 | 22.54±1.11 | 10.94% | 71.4% |
| 11 | 19.62±1.09 | 12.95% | 75.11% |
| 12 | 17.25±1.06 | 12.24% | 78.11% |
| 24 | 2.2±0.22 | 87.24% | 97.2% |
Table 2: Table showing statistically significant (p<0.05) value between each interval.
| Time Interval | p-value |
| 0-1 | <0.0001 |
| 1-2 | <0.0001 |
| 2-3 | <0.0001 |
| 3-4 | <0.0001 |
| 4-5 | <0.0001 |
| 5-6 | 0.0003 |
| 6-7 | 0.0001 |
| 7-8 | 0.0157 |
| 8-9 | 0.0059 |
| 9-10 | 0.0093 |
| 10-11 | 0.04109 |
| 11-12 | 0.0259 |
| 12-24 | <0.0001 |
This is the first study conducted to detect effect of delayed serum separation on glucose concentration of dogs at room temperature in Nepal to the best of our knowledge. (Sunderman et al., 1956) stated that glucose decreases at the rate of 5 to 10 mg/dl/hr. which supports our finding where there is decline serum glucose concentration at the rate of 6.8 mg/dl/hr. However, (McMillin, 1990) stated that rate of glucose decline is about 8 to 10% per hour in most species including dog at 22°C.This slight lower decline glucose rate may be due to the temperature difference. So, this means that with increasing temperature rate of glucose decline may also increase. Statistically significant may be sometimes medically irrelevant. As in our research there is glucose decline at rate of 10.8% /hr. which was statically significant as well but this may be medically unimportant. Suppose glucose value of a dog at 0 hour was found 100 mg/dl. If the sample is centrifuged after an hour post blood collection the glucose decline will be about 10.8 mg/dl and the glucose value will be about 89.2mg/dl. Now both of the values are within normal range and won’t affect the diagnosis. However, this may not be always the case especially when the value is in borderline.
Conclusion
This study shows that glucose decreases rapidly with time if blood sample is stored at room temperature. Average rate of glucose decline is 10.8% per hour. Based on the results of this study serum should be separated as soon as possible to get accurate glucose value. Even one-hour delay can be considered for measuring the glucose value if the glucose value is not in the borderline. So, it is better to measure the glucose value as soon as blood is collected.
Acknowledgment
To all frontline workers in the fight against the pandemic COVID-19. Also, we like to acknowledge Dr. Tulsi Ram Gompo, Dr. Awadesh Jha, Dr. Anil Koirala, Dr. Laxman Bhatta, Dr. Ankita Shrestha and Dr. Bishal Maharjan for their valuable suggestions and guidelines during the research and manuscript preparation.
Author Contributions
Conceptualization: Bishal Chand, Deepak Subedi, SK Poudel. Research Conduct: Bishal Chand, SK Poudel
Data analysis: Bishal Chand. Writing original draft: Bishal Chand, Deepak Subedi. Review and editing: Bishal Chand, Deepak Subedi, SK Poudel.
Conflict of interest
The Authors have declared no conflict of interest.
References






