Advances in Animal and Veterinary Sciences
Research Article
Isolation and Identification of Parasites From Housefly (Musca domestica) in Mosul City, Iraq
Reedha N. Hamoo*, Aseel I. Alnuri
Department of Biology, College of Education for Girls, University of Mosul, Mosul, Iraq.
Abstract | Houseflies (Musca domestica) considered the most predominant vector of zoonotic parasitic diseases of human and domestic animals. This study was conducted to determine the prevalence of parasites and their kind that could be transmitted mechanically by flies. A total of 140 houseflies were randomly collected from butcher markets, sweet markets, toilets of schools, and groceries in Mosul city, Iraq, from August to October 2017. The results revealed the existence of 11 types of parasites. Entamoeba histolytica and Entamoeba coli have predominant percentage of 17.02% for each one, whereas the least prevalence was Isospora spp. (2.1%) of total investigated parasites. Other parasites, Cryptosporidium, Giardia Lamblia, Fasciola hepatica, and others have various percentages. It was concluded that houseflies are still public health trouble and it should be noted that climate changes in Mosul city at summer, go together with the bad drainage of sewage and accumulated rubbish, has a key role in the widespread of houseflies.
Keywords | Housefly, Parasites, Mechanical transmission, Musca domestica, Mosul, Iraq.
Received | May 11, 2019; Accepted | June 07, 2019; Published | June 30, 2019
*Correspondence | Reedha N Hamoo, Department of Biology, College of Education for Girls, University of Mosul, Mosul, Iraq; Email: [email protected]
Citation | Hamoo RN, Alnuri AI (2019). Isolation and identification of parasites from housefly (musca domestica) in mosul city, iraq. Adv. Anim. Vet. Sci. 7(8): 711-714.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.8.711.714
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Hamoo and Alnuri. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The housefly, Musca domestica, as a part of arthropods, is supposed to have developed in the Cenozoic era, perhaps in the Middle East, and has been extended over the world as a human commensal. Fly consider the most prevalent vector of parasitic diseases of man and animals (El-Sherbini and Gneidy, 2012). In the tropic, housefly associated with unsanitary areas, and can exist in both rural and urban zones of tropical and hot climates (Hussein and John, 2014). Likewise, Musca domestica abundantly grows in the fields of human activities like abattoir, poultry and animal farmhouses, food markets, and hospitals (Iqbal et al., 2014).
Countless pathogenic microorganisms can be transmitted mechanically by housefly, triggering fatal complaints as anthrax, leprosy, tuberculosis, typhoid fever, dysentery, and various intestinal parasites (Graczyk et al., 2001; Al-Aredhi, 2015). In addition, this arthropod contributed to the transmission of various illnesses-causing factors of human, domestic animals, and birds (Graczyk et al., 1999; Hewitt 2011). Stimulatingly, Nassiri et al. (2015) believed that the characteristics and kinds of pathogenic agents carried by houseflies depended on the area of an insect where collected. Mechanical transmission of pathogenic agents may be the most critical one among the other routes as via feces or saliva of the flies. On the other hand, an Egyptian study revealed more than 25% of investigating flies were contaminated with various species of parasites like E. histolytica, C. parvum, B. coli, A. lumbricoides, A. doudunale, E. vermicularis, T. trichura, and S. stercoralis (El-Sherbini and Gneidy, 2012).
In a recent review study, Khamesipour et al. (2018) demonstrated previous project deals with more than ten types of parasites with medical and/or veterinary significance, that could be transmitted by houseflies in different parts of the world, with the wide prevalence percentage (5-62%) depending on the species of parasites. According to the kno-
Table 1: Prevalence of parasites, number encountered (%), isolated from external surface of houseflies.
| Parasite species | Entam- oeba histolytica |
Entam- oeba coli |
cryptos- poridium |
Giardia Lamblia | Isospora spp. | Hymen- olepsis nana |
Fasciola hepatica | Taenia spp. | Entro- bisv ermicu laris |
Toxo-cara spp. |
Ascaris spp. | Total (%) |
| Locations | ||||||||||||
| Butcher markets | 4 (21) | 1 (5.2) | 3 (15.7) | - | 1 (5.2) | - | 3 (15.7) | 2 (10.5) | 1 (5.2) | 2 (10.5) | 2 (10.5) | 19 (40.4) |
| Toilets of schools | 2 (20) | 2 (20) | 1 (10) | 2 (20) | - | - | - | - | 3 (30) | - | - | 10 (21.2) |
| Groceries | 2 (16.6) | 3 (25) | - | 1 (8.3) | - | 1 (8.3) | 2 (16.6) | - | 2 (16.6) | - | 1 (8.3) |
12 (25.5) |
| Sweet markets | - | 2 (33.3) | 1 (16.6) | - | - | 1 (16.6) | - | 1 (16.6) | 1 (16.6) | - | - | 6 (12.7) |
| Total (%) | 8 (17.02) | 8 (17.02) | 5 (10.6) | 3 (6.3) | 1 (2.1) | 2 (4.2) | 5 (10.6) | 3 (6.3) | 7 (14.8) | 2 (4.2) | 3 (6.3) |
47 (100) |
wledge of the authors, there was no definitive study to investigate species of parasites carried by housefly, at least in Mosul city, Iraq. Therefore, the aim of this study was concerned with the isolation and identification of various pathogenic parasites which was brought by a local housefly.
Materials and Methods
Collection of Flies
This project was conducted from August to October 2017, in the Mosul city (36.34°N 43.13°E), Iraq. One hundred and forty samples of adult houseflies (Musca domestica) were got from different places; butcher markets, sweet markets, toilets of schools, and groceries.The houseflies were collected randomly using sweep net over a surface where houseflies stopped at, then flies were unconstrained into labeled constructed boxes (Ahmadu et al., 2016).
Isolation of External Parasites
Collected flies transferred into labeled sterile specimen bottles that carried information such as date and location of the isolate. Killed flies by deep freezing were immersed in 5 ml of normal saline in the sterile test tube ,and gently shacked for three minutes, immediately the solution was transferred into conical tubes and centrifuged at 3000 rpm for 5 minutes (Fotedar et al., 1992).The supernatant discarded and the sediment positioned on free glass slides stained with Lugol’s iodine stain and examined by light microscope (Nwangwu, 2013;Al-Aredhi, 2015).
Identification of Parasites
Species and group of parasites were identified according to their shape, size, and peculiar structures (Urquhart et al., 1996).
Statistical Analysis
Statistical analysis included determination of the total and percentage of parasites depending on the total number, using Microsoft Excel software (2010) (Bass, 2007).
Table 2: Distribution of the kind of parasites on the studied locations.
| Sample sources | No. of examined flies | No. of infested flies (%) | No. of protozoa (%) | No. of helminthes (%) |
| Butcher markets | 35 | 19 (40.4) | 9 (47.3) | 10 (52.6) |
| Toilets of schools | 35 | 10 (28.5) | 7 (70) | 3 (30) |
| Groceries | 35 | 12 (34.2) | 6 (50) | 6 (50) |
| Sweet markets | 35 | 6 (17.1) | 3 (50) | 3 (50) |
| Total (%) | 140 | 47 (33.5) | 25 (53.1) |
22 (46.8) |
Results
A total of 140 houseflies were randomly collected, an external surface of Musca domestica was examined for parasites. Table 1 shows the isolation of 11 species of parasites that isolated from houseflies in Mosul city. Entamoeba histolytica cyst which was spheroidal compact with four nuclei (a diameter of about 20 µm), and Entamoeba coli that was larger than E. histolytica cyst with predominant 6-8 nuclei, were a dominant percentage (17.02%) for each one, whereas the least prevalence was to Isospora spp. (2.1%) of total investigated parasites. However, according to the location of collection, butcher markets seem to be highly contaminated (40.4%) by parasites isolated from flies, in which E. histolytica represent 21% of whole parasites isolated from this location, followed by groceries (25.5%), where an E. coli was the predominant parasite (25%) of this site. However, sweet markets have 12.7% of parasitic contamination, in which an E. coli was also the main parasite (33.3%) of this location. Eggs of E. vermicularis were the major parasite (30%) that isolated from flies of school toilet. On the other hand, findings reveal that the protozoan parasites were greater (53.2%) than eggs of helminthes (46.8) (Table 2) such as Ascaris lumbricoides eggs had a round form with outer shell, while hookworm eggs had oval shape enclosing embryo. Whereas, Figure 1 shows various eggs and sacs of different kinds of the isolated parasites.
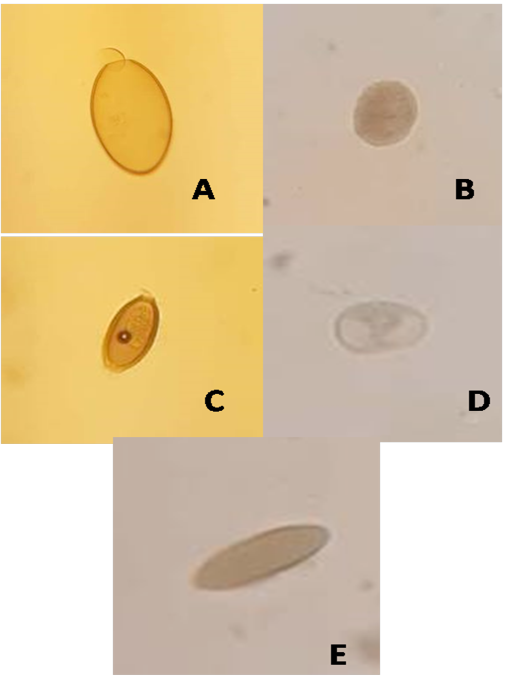
Figure 1: Eggs and sacs of certain parasites isolated from houseflies: (A)Fasciola egg (B)Blantidium coli sac (C)Enterobius egg (D)Entamoeba coli sac (E)Ascaris egg.
Discussion
A mechanical spread of pathogenic agents is critical for human and animal health, so, the housefly is one of the well-known disease transmitter (Graczyk, et al., 2005). Flies have been confirmed to transmit parasites by their mouthparts, via vomits, through feces, and by their whole body surface (Forster et al., 2009). Certain biological characters of this insect, like a hairy and sticky exoskeleton (Urquhart et al., 1996), and feeding behavior that depends on the vomiting before licking of the food (Sales et al., 2002; Forster et al., 2009), widely promote the transmission process. On the other hand, housefly considers more close arthropod of man and his domestic animals, thus play a key role in disease transmission. Also, as fly lives among human diet and waste, the problem becomes more challenging (Gerald et al., 1998). As illustrated in the results, there was a significant difference in the percentage of isolated parasites according to the location, in which butcher markets have higher rate (40.4%), and this may be due to that these locations were attracting focus because of their high humidity, and presence of slaughtered animal body parts, feces, viscera and blood (Gerald et al., 1998; Ahmadu et al., 2016). Frequently reported parasites from housefly belonged to the genera; Ascaris, Entamoeba, and Entrobius, where that have great medical and veterinary significance causing enteric diseases like amoebiasis which documented as the worldwide second lethal parasitic disease (Nayduchand Burrus, 2017).
However, butcher markets have various cysts of protozoa and eggs of helminthes as Fasciola hepatica, also Toxocara spp., this may be due to the existence of carrier animals, like stray dogs and cats, near the butchers markets, this agrees with Al-Aredhi, (2013) who isolated different parasites transmitted by houseflies in various locations of Al-Diwaniya city, Iraq.
Our results also demonstrated that flies isolated from grocery markets have a higher rate of parasites (25.5%) and this may be due to the suitable environment to housefly mainly the existence of organic substances. On the other hand, our results revealed the protozoan parasite percentage (53.2%) was higher than eggs of helminthes (46.8%).
It should be noted that climate changes and higher temperatures, particularly in our city Mosul in summer, accompanied by bad drainage of sewage and accumulated rubbish, has a key role in the widespread of houseflies. Thus, this study could be considered a pilot that must progress in the future to uncover the dangerous role of houseflies in the of various etiological outbreaks of diseases.
Acknowledgements
Authors would like to thanks College of Education for Girls, University of Mosul, Mosul, Iraq for the support of this work.
Conflict of interest
There is no conflict of interest.
Authors Contribution
Reedha N Hamoo designed the experiments and analyzed the data. Aseel Alnuri collected samples and analyzed the data and both authors carried out the experiments and wrote the manuscript.
References






