Advances in Animal and Veterinary Sciences
Research Article
Ovine Theileriosis: Clinical, Pathological and Molecular Investigations
Mahmoud Eliwa1, Khaled Mohamed Ahmed Mahran2*, Waheed Ali Mousa3, Naglaa Hagag1, Mohamed Ibrahim Shaalan4, Mostafa Mahmoud Bashandy2
1Genome Research Unit, Animal Health Research Institute (AHRI), Dokki, Egypt; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 3Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 4Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Abstract | Ovine theileriosis is a hemoprotazoan infection transmitted by tick bites which cause severe economic loss. This study aimed to survey Theileria infection of sheep in different localities of Egypt (Cairo, Giza and Al Monofia) by using microscopic and molecular detection associated with clinicopathological and pathological investigations. A total of 152 sheep blood samples were selected from farms and veterinary clinics. Microscopic examination of blood smears revealed that the incidence of Theileria infection was 21.7% while the incidence was 36.8% using PCR assay (universal Theileria spp. Primer). The use of species-specific primers showed a result of 53.6% single infection; Theileria ovis, and 46.4% mixed infection; T. ovis and Theileria lestoquardi. According to the results of PCR, animals were divided into 3 groups: Theileria negative group, T. ovis group and mixed T. ovis and T. lestoquardi group. The examination of both infected groups revealed non-significant changes between them. The hemogram revealed significant macrocytic hypochromic anemia, leukopenia, neutropenia, lymphopenia, monocytopenia, eosinopenia and thrombocytopenia in Theileria infected groups compared with Theileria negative group. Biochemical analysis revealed significant hypoproteinemia, hypoalbuminemia, total and indirect hyperbilirubinemia with elevations of AST and GGT activities with increase in concentration of BUN and creatinine while no significant changes in A:G ratio values and direct bilirubin concentration in Theileria infected groups compared with Theileria negative group. The pathological investigation revealed lymphocytic depletion and necrosis with hemorrhages in lymph node and spleen. Sequencing and phylogenetic analysis were performed by targeting the 18S rRNA gene of Theileria spp. In conclusion, according to our knowledge, this is the first report of phylogeny of T. lestoquardi infected sheep in Egypt.
Keywords | Sheep, Theileriosis, Clinicopathological, Molecular investigations
Received | September 28, 2020; Accepted | December 04, 2020; Published | February 15, 2021
*Correspondence | Khaled M.A. Mahran, Department of Clinical Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; Email: [email protected]
Citation | Eliwa M, Mahran KMA, Mousa WA, Hagag N, Shaalan MI, Bashandy MM (2021). Ovine theileriosis: Clinical, pathological and molecular investigations. Adv. Anim. Vet. Sci. 9(4): 462-472.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.462.472
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Eliwa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Ovine theileriosis is one of the most important hemoprotazoan infections transmitted by tick bites (Zhao et al., 2019) which hinder the development and productivity of livestock industry, and result in severe economic losses as reduced productivity, high cost of treatment and the death of infected animals (Zhang et al., 2014; Elsify et al., 2015). Theileria is a genus belonging to Theileriidae family in Piroplasmida order. This order belongs to the Apicomplexa phylum (Levine, 1985).
The ovine theileriosis is a worldwide disease that is reported in different geographical areas including the Middle East, East and North Africa, India, China, Central Asia, and Eastern and Southern Europe (Razmi et al., 2019). In Egypt, the Theileria was first discovered in 1914 (Littlewood, 1915). The incidence of ovine theileriosis by microscopic examination was 33.75% in different 4 governorates (El-Fayoum, Giza, Cairo and El-Gharbia) (Radwan and El–Kelesh, 2009), 77.93% in Halayeb and Shalatin (Fadel et al., 2010) and 15.56% in Giza (Hegab et al., 2016).
The predominant clinical signs of ovine theileriosis are pyrexia, loss of appetite, gross enlargement of prescapular lymph nodes, pale mucous membranes, lacrimation and presence of ticks or tick bites on various parts of the body (Çöl and Uslu, 2006; Al-Fetly, 2012).
Diagnosis of theileriosis is mainly based on clinical signs in infected animals, and microscopic examination of stained thin blood and lymph node smears. In addition, Theileria species can be differentiated by using serological assays as enzyme-linked immunosorbent assay (ELISA), and molecular techniques such as polymerase chain reaction (PCR) (Bakheit et al., 2006; Youssef et al., 2015). PCR product may be sequenced to confirm the identity of the parasite (AL-Hosary et al., 2015; Rjeibi et al., 2016).
Hematological and biochemical alterations during Theileria infection in sheep were reported in several studies as result of multiple organs affection causing numerous gross and microscopic pathological changes (Akhter et al., 2017). These alterations provide some information about the severity and pathogenicity of the disease, and guide for the differential diagnosis and prognosis of the disease condition (Çöl and Uslu, 2006; Al-Fetly, 2012; Yaghfoori et al., 2017).
This study aims to survey the prevalence of ovine theileriosis in certain areas of Egypt (Cairo, Giza and Al Monofia), and to demonstrate the hematological, biochemical and pathological changes during Theileria infection with the identification of Theileria species by molecular techniques such as PCR, sequencing and phylogenetic analysis.
MATERIALS AND METHODS
Animals subjected to study
A total of 152 sheep were subjected to general examination and sampling in different localities of Cairo, Giza and Al Monofia (Table 1). The animals were randomly selected from farms, slaughterhouses and veterinary clinics. The animals were divided into 3 groups according to PCR result: negative group (96 sheep), T. ovis infected group (30 sheep) and mixed infection with T. ovis and T. lestoquardi group (26 sheep). This study was approved (VetCU10102019091) from the Institutional Animal Care and Use Committee (IACUC) of Faculty of Veterinary Medicine, Cairo University, Egypt.
Clinical examination of animals
All sheep, under the study, were subjected to the general clinical examination. Moreover, they were examined for the presence of ticks or tick bites, anemic mucous membrane and enlarged lymph nodes were also considered.
Table 1: Numbers of examined sheep according to different geographical localities.
| Location | Number of sheep |
| 1. Badr center (Al Monofia) | 29 |
| 2. Bahtim and Marg (Cairo) | 47 |
| 3. Kafr Tohormous and Faisal (Giza) | 29 |
| 4. Al Oubour (Cairo) | 13 |
| 5. Al-Saff (Giza) | 34 |
| Total | 152 |
Samples collection
Blood and serum collection
The blood samples were collected from jugular vein by a sterile needle. The samples were divided into sterile air-vacuumed tubes with potassium salt of ethylene diamine tetra acetic acid (EDTA) as anticoagulant for hematological and molecular examination and into plane tubes for serum collection for biochemical examination.
Lymph node biopsy
The prescapular lymph nodes biopsies were aspirated by fine needle aspiration after sterilization of sampling site (Almeria et al., 2001).
Organ tissue specimens
Swollen lymph nodes and enlarged spleen were collected just after slaughter of the animals in the slaughterhouse and preserved in 10% neutral buffered formalin, for histopathological examination (Suvarna et al., 2012).
Microscopic smear examination
Blood and lymph node smears were prepared on clean slide, air dried, fixed by methanol and stained with Field stain (Soulsby, 1982). The blood smears were examined microscopically to detect intra-erythrocytic merozoites and schizonts in lymphocytes, while the lymph node smears were examined to detect the schizonts (Koch’s blue bodies) inside the lymphocytes.
Polymerase chain reaction (PCR)
The DNA of Theileria was extracted from collected whole blood by using Thermo Scientific GeneJET Genomic® extraction kit (Life Technologies Inc., Carlsbad, California, USA) according to the manufacturer’s instructions. The used primers are listed in Table 2 as universal Theileria spp. (Allsopp et al., 1993) and specific primers; T. lestoquardi (Kirvar et al., 1998) and T. ovis (Altay et al., 2005). A universal Theileria spp. primer was used for all collected blood samples while the specific primers were only applied on the positive samples resulted from universal Theileria spp. primer. The PCR amplifications were performed in total volume of 25 µl containing 6.5 µL of template DNA, 12.5 µl of Thermo Scientific® 2× PCR Master mix (Life Technologies Inc., Carlsbad, California, USA), and 3 µl of each forward and reverse primer (20 pmol/µl). The PCR reactions were carried out in a thermocycler (BIO-RAD®, Singapore). The PCR cycling program were done according to Riaz and Tasawar (2017) as; initial denaturation at 94°C for 15 min for 1 cycle followed by denaturation, annealing and extension for 35 cycles at temperature (94, 60 and 72°C, respectively). At last final extension at 72°C for 7 min in 1 cycle. The PCR products were checked on 1.5% agarose gel stained with ethidium bromide, visualized and photographed using a gel document system (BIO-RAD®, Singapore).
Clinicopathological examination
Hematological studies
Hematological studies were performed by using Exigo vet® hematology analyzer on whole blood samples. The examination included RBCs count, hematocrit (Hct), hemoglobin (Hb) concentration, red cell indices [mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC)], total leukocyte count (TLC) and platelet count. Differential leukocyte count (DLC) were performed manually by the method described by Weiss and Wardrop (2010).
Biochemical analysis
The biochemical examination was performed on serum samples by using commercial biochemical diagnostic kits provided by Vitro Scient®, Egypt. The biochemical parameters included total proteins (Doumas et al., 1981), albumin (Doumas et al., 1971), globulin, A/G ratio, aspartate aminotransferase (AST) (Reitman and Frankel, 1957), gamma-glutamyl transpeptidase (GGT) (Persijn and van der Slike, 1982; Szasz, 1969), total, direct and indirect bilirubin (Jendrassik and Grof, 1938), blood urea nitrogen (BUN) (Sampson et al., 1980) and creatinine (Jaffé, 1986).
Histopathological examination
The collected tissues were routinely processed and stained with hematoxylin and eosin (H and E) for microscopic examination (Suvarna et al., 2012). Additionally, spleen smears were stained with Prussian blue stain to demonstrate the hemosiderin pigment deposition (Cluzel et al., 2016).
Sequencing and phylogenetic analysis
The gene-specific PCR amplicons of the 18S rRNA gene of T. ovis and T. lestoquardi were size-separated using agarose gel electrophoresis, excised and purified from gels using the QIAquick® Gel Extraction Kit (Qiagen, Germany). Further, purified products from gel were used directly for cycle sequencing reactions using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA). Products of cycle sequencing reactions were purified using DyeEx® Spin Kit (Qiagen, Germany) and sequenced on an ABI 3500XL® Genetic Analyzer (Life Technologies, USA). The obtained sequences of T. lestoquardi and T. ovis were uploaded at GenBank and given the accession numbers; MT002826 and MT002827, respectively. The 18S rRNA genes of MT002826 and MT002827 sequences were assembled, aligned and edited using the Bioedit® software, version 7.2.5 (Hall, 1999). A Basic Local Alignment Search Tool (BLAST) was used on the National Centre for Biotechnology Information (NCBI) platform. Phylogenetic trees were constructed by using molecular evolutionary genetics analysis (MEGA®) software version 10.1.7 (Kumar et al., 2018) on the bases of maximum likelihood methodology (Hasegawa et al., 1985).
Table 2: Primers design of different Theileria primers sets.
| Primer | Target gene | Primer specificity | Product size (bp) |
| Universal spp. | 18S rRNA |
F.5'-AGTTTCTGACCTATCAG-3' R.5'-TTGCCTTAAACTTCCTTG-3' |
1098 |
|
Specific T.ovis |
18S rRNA |
F. 5'-TCGAGACCTTCGGGT-3 R.5'-TCCGGACATTGTAAAACAAA-3' |
520 |
|
Specific T.lestoquardi |
18S rRNA |
F. 5'-GTGCCGCAAGTGAGTCA-3' R.5'GGACTGATGAGAAGACGATGAG3' |
785 |
Statistical analysis
The data were expressed as mean ± standard deviation (SD). Analysis of variance was performed by one-way ANOVA using SPSS version 25.0 for windows (Statistical Package for the Social Sciences; SPSS Inc., Chicago, USA).
RESULTS
Clinical signs
Clinical manifestations of ovine theileriosis in infected sheep and their incidence were listed in Table 3.
Table 3: The incidence of clinical signs of theileriosis in sheep.
| Clinical signs | Number of sheep |
| Fever | 39 |
| Cachexia and emaciation | 33 |
| Lacrimation and conjunctivitis | 28 |
| Pale mucous membrane | 26 |
| Tick bite wound | 10 |
Prevalence of theileria infection
The microscopic examination of blood smears revealed the presence of intracellular forms of Theileria piroplasms and schizonts in 33 out of 152 samples (21.7%) while its incidence by using PCR (universal Theileria spp. Primer) was 56 out of 152 (36.8%) of examined animals. Moreover, the use of microscopic examination of blood smears and PCR assay revealed different prevalence in the different localities from which the samples were collected, Table 4.
Table 4: The prevalence of ovine theileriosis in the different localities.
| Location | Number of sheep | Diagnostic techniques | |||
| Blood smears | PCR | ||||
| 1. Bahtim and marg (Cairo) | 47 | 2 | 4.3% | 14 | 29.8% |
| 2. Al Oubour (Cairo) | 13 | 0 | 0.0% | 0 | 0.0% |
| 3. Kafr tohormous and faisal (Giza) | 29 | 12 | 41.4% | 13 | 44.8% |
| 4. Al-Saff (Giza) | 34 | 18 | 52.9% | 28 | 82.4% |
| 5. Badr center (Al Monofia) | 29 | 1 | 3.4% | 1 | 3.4% |
| Total | 152 | 33 | 21.7% | 56 | 36.8% |
Microscopic examination
The microscopic examination of blood smear revealed pleomorphic (round, oval, rod and comma) piroplasms (Figures 1 and 2). They were observed as individual (one piroplasm per erythrocyte), double, triple and tetra-forms. Moreover, the microschizonts were observed in the lymphocytes of blood smear (Figure 3) while the macroschizonts (Koch’s blue bodies) were demonstrated in the lymphocytes of lymph nodes smears (Figure 4). The infected RBCs showed crenation, spherocytosis and acanthocytosis.
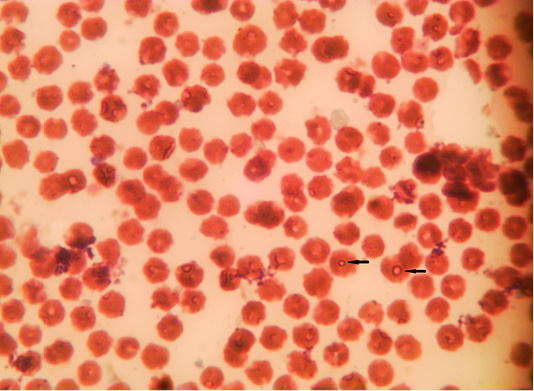
Figure 1: Microphotograph showing intra-erythrocytic merozoits of sheep blood smears (Field stain, x100).
Polymerase chain reaction assay (PCR)
The use of specific primers assured the presence of T. ovis and T. lestoquardi in the different localities, Table 5. However, T. lestoquardi was only identified in association of T. ovis (mixed infection) in Al-Saff (Giza) group. The product size of universal Theileria, T. lestoquardi and T. ovis were 1098bp (Figure 5), 785 bp (Figure 7) and 520 bp (Figure 6), respectively.
Table 5: Prevalence of Theileria infections with different species.
| Infection | Number of sheep | Infection (%) |
| T. ovis | 30 | 53.6% |
|
T. lestoquardi + T. ovis (mixed) |
26 | 46.4% |
| Total | 56 | 100% |
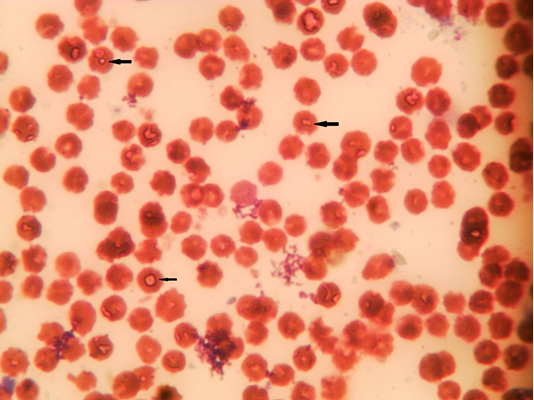
Figure 2: Microphotograph showing intra-erythrocytic merozoits of sheep blood smears (Field stain, x100).
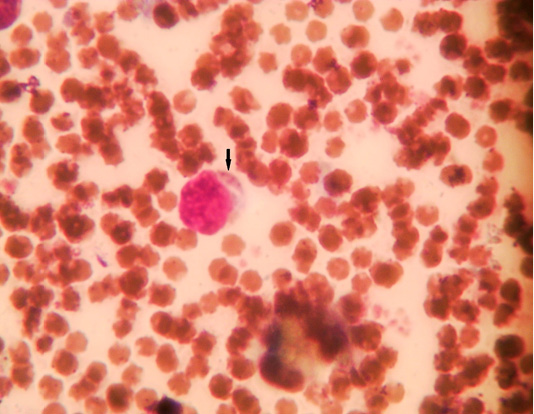
Figure 3: Microphotograph showing lymphocytic microschizont of sheep blood smears (Field stain, x100).
Hematological changes
There was significant decrease in RBCs count, hematocrit, Hb concentration, MCHC, TLC, neutrophils, lymphocytes, monocytes, eosinophils, and platelets with significant increase of MCV in infected groups compared with Theileria negative group (Table 6). There were no significant differences between T. ovis group and mixed Theileria group.
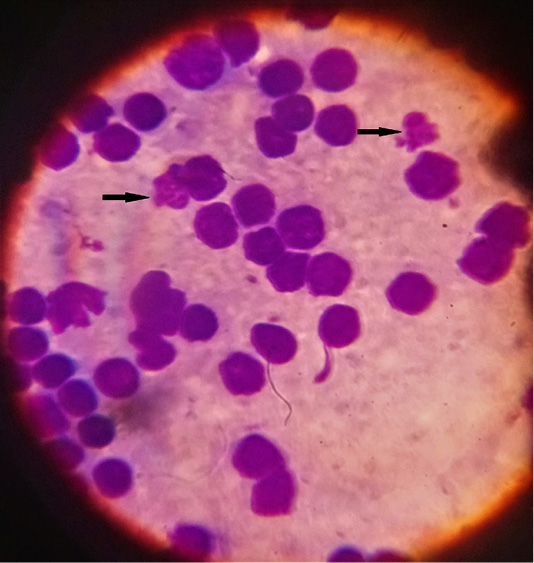
Figure 4: Microphotograph showing lymphocytic macroschizonts (Koch’s blue bodies) of lymph nodes smears (Field stain, x100).
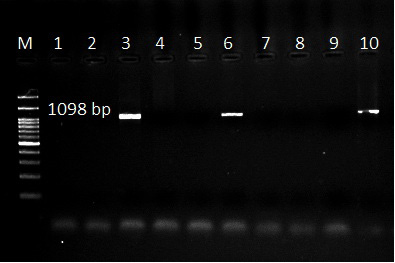
Figure 5: Ethidium bromide stained gel electrophoresis showing specific bands from PCR amplified products by using Theileria spp. universal primer in lane 3,6 and 10. Lane M: marker 100 bp.
Biochemical changes
Significant increases in AST, GGT, total bilirubin, indirect bilirubin, urea, and creatinine with significant decreases in total proteins and albumin were recorded in infected groups in comparison with Theileria negative group. Moreover, there was no significant change in the values of A:G ratio and direct bilirubin (Table 7). There were no significant differences between T. ovis group and mixed Theileria group.
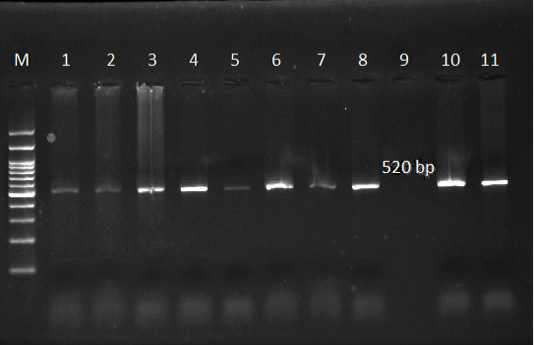
Figure 6: Ethidium bromide stained gel electrophoresis showing specific bands from PCR amplified products by using T. ovis specific primer in lane 1, 2, 3, 4, 5, 6, 7, 8, 10 and 11. Lane M: marker 100 bp.
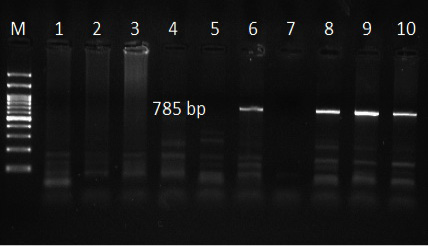
Figure 7: Ethidium bromide stained gel electrophoresis showing specific bands from PCR amplified products by using T. lestoquardi specific primer in lane 6, 8, 9 and 10. Lane M: marker 100 bp.
Table 6: Hematological parameters of Theileria infected and control groups.
| Parameter | Unit |
Theileria negative group |
Theileria ovis group |
Mixed Theileria group |
| RBCs count |
×106 cell/μl |
9.84±0.88a |
6.72±0.76b |
6.62±0.33b |
| Hct | % |
29.25±1.12a |
22.97±1.94b |
21.81±1.26b |
| Hb | g/dl |
10.21±0.91a |
7.11±0.54b |
6.88±0.39b |
| MCV | fl |
29.72±3.17a |
34.27±2.51b |
33.01±2.60b |
| MCHC | % |
33.86±1.54a |
30.83±1.26b |
31.44±1.08b |
| TLC |
×103 cell/μl |
14.98±1.32a |
9.51±0.84b |
6.65±0.26b |
| Neutrophils |
×103 cell/μl |
5.05±0.27a |
4.31±0.19b |
3.58±0.14b |
| Lymphocytes |
×103 cell/μl |
8.08±0.81a |
4.29±0.25b |
2.41±0.19b |
| Monocytes |
×103 cell/μl |
1.493±0.088a |
0.755±0.033b |
0.528±0.027b |
| Eosinophils |
×103 cell/μl |
0.356±0.056a |
0.151±0.016b |
0.124±0.026b |
| Platelets |
×103 cell/μl |
286.58±13.55a |
202.11±15.81b |
188.47±9.25b |
Different superscripts in the same raw refer to significant changes (p<0.05).
Table 7: Biochemical parameters of Theileria infected and control groups.
| Group | Unit |
Theileria negative group |
Theileria ovis group |
Mixed Theileria group |
| Total proteins | g/dl |
7.98±0.47a |
6.48±0.69b |
6.73±0.41b |
| Albumin | g/dl |
3.82±0.15a |
3.29±0.33b |
3.25±0.28b |
| Globulin | g/dl |
4.16±0.38a |
3.19±0.17b |
3.48±0.72b |
| A:G ratio |
1.51±0.099a |
1.68±0.076a |
1.42±0.088a |
|
| AST | U/L |
26.21±3.96a |
45.63±2.52b |
65.25±5.56b |
| GGT | U/L |
24.13±1.57a |
41.99±1.49b |
40.33±1.01b |
| Total bilirubin | mg/dl |
0.930±0.052a |
1.309±0.065b |
1.118±0.021b |
| Direct bilirubin | mg/dl |
0.608±0.054a |
0.573±0.038a |
0.618±0.012a |
| Indirect bilirubin | mg/dl |
0.624±0.061a |
1.002±0.035b |
0.997±0.021b |
| Urea | mg/dl |
23.16±1.74a |
46.92±1.27b |
50.53±1.10b |
| Creatinine | mg/dl |
0.983±0.178a |
1.609±0.209b |
1.346±0.124b |
Different superscripts in the same raw refer to significant changes (p<0.05).
Macroscopic and histopathological changes
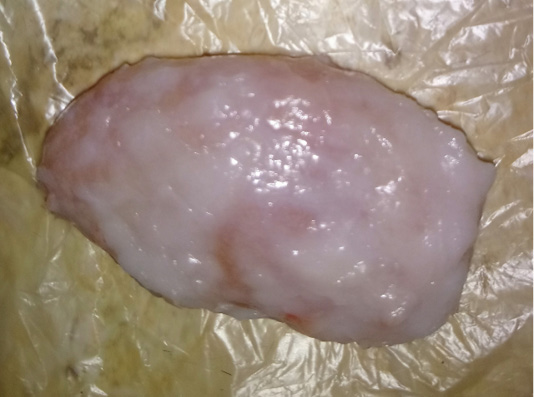
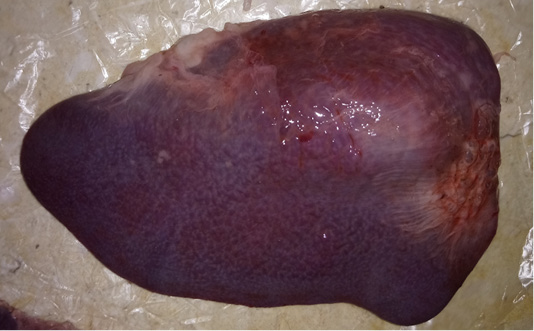
The examined organs (spleen and lymph nodes) showed severe enlargement with sub-capsular hemorrhages (Figures 8 and 9). Meanwhile, the microscopic examination of spleen revealed diffuse depletion of the lymphoid tissue, degenerative changes in the septal connective tissue and multiple necrotic foci with diffuse hemorrhages accompanied with deposition of hemosiderin pigment (Figure 10). Moreover, depletion and necrosis of lymphoid follicles were observed in the lymph nodes (Figure 11).
Phylogenetic analysis
The comparison of the18S rRNA T. Lestoquardi partial sequence (MT002826) revealed 100% homology with T. lestoquardi from bovine/Egypt (KF771185), ovine/Tunisia (KM117212 and KJ458988), ovine/China (AF081135) and ovine/Iraq (KC778786). Also, there was a 99% homology with the bovine T. annulata from France (KF429799), Turkey (AY508463), Pakistan (MG599090) and Spain (DQ287944) (Figure 12) while the 18S rRNA T. ovis partial sequence (MT002827) revealed 100% homology with the ovine T. ovis from South Africa (MG203885), France (EU622911), Turkey (KT851436), China (FJ603460), Tanzania (MG725961) and India (MH819508) (Figure 13).
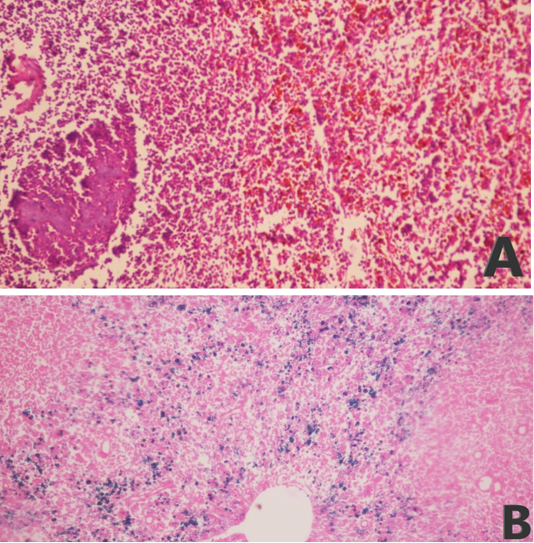
Figure 10: Microphotograph of sheep spleen showing (A) diffuse hemorrhage with deposition of brownish hemosiderin pigment in addition to necrotic foci and diffuse depletion of the lymphoid tissue (H and E, x200) and (B) diffuse deposition of bluish hemosiderin pigment (Prussian blue, x200).
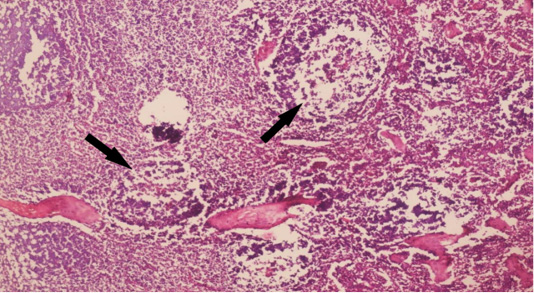
Figure 11:Microphotograph of sheep lymph node showing depletion and necrosis of multiple lymphoid follicles (arrows) (H and E, x200).
DISCUSSION
Ovine theileriosis is an important hemoprotazoan disease of sheep causing severe economic losses as reduced productivity and sometimes cause high mortality of infected animals (Zhao et al., 2019). In this study, the ovine theileriosis infected animals showed the common clinical signs of the disease as pyrexia, cachexia, emaciation, lacrimation, conjunctivitis, pale mucous membrane and tick bite wounds.
Microscopic examinations of blood smears, prepared from the animals of this study, revealed that the incidences of Theileria species were 4.3%, 0.0%, 41.4%, 52.9% and 3.4% in Bahtim and Marg, Al Oubour, Kafr Tohormous and Faisal, Al-Saff, and Badr center; respectively, with an overall incidence of 21.7% while by using PCR (universal Theileria spp. primer) the incidences were 29.8%, 0.0%, 44.8%, 82.4% and 3.4% in the same localities with an overall incidence of 36.8%. These results are approximately similar to the results of Hegab et al. (2016) who reported a prevalence of 17.08% by using microscopic examination of blood smears and 40% by using PCR in the resident sheep samples collected from different localities of Egypt.
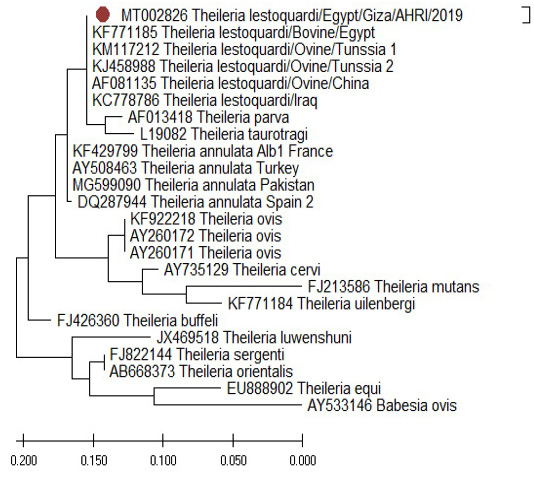
Figure 12: Phylogenetic tree of T. lestoquardi (MT002826) revealed 100% homology with T. lestoquardi from (KF771185), (KM117212 and KJ458988), (AF081135) and (KC778786). This isolate showed 99% homology with T. annulata from (KF429799), (AY508463), (MG599090) and (DQ287944).
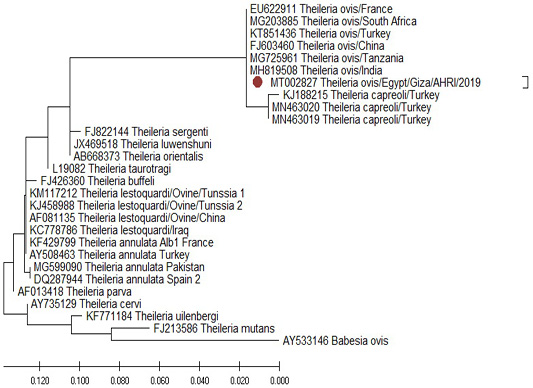
Figure 13: Phylogenetic tree of T. ovis (MT002827) revealed 98% homology with T. ovis from (MG203885), (EU622911), (KT851436), (FJ603460), (MG725961) and (MH819508).
According to the results of PCR (by using universal and spp. specific primers), the animals of this study, were divided into 3 groups: Theileria negative group, T. ovis group and mixed T. ovis and T. lestoquardi group. The clinic pathological examination of the animals of the groups under the study revealed significant changes of some parameters in both infected groups in comparison with the Theileria negative group. Moreover, the significance was more prominent in the mixed group than the T. ovis group due to there is co-infection with T. lestoquardi and T. ovis.
The microscopic examination of blood smears of infected animals showed pleomorphic intra-erythrocytic piroplasms and microschizont inside lymphocytes while the lymph node smears showed macroschizont (Koch’s blue bodies) inside the lymphocytes. These microscopic findings are similar to Çöl and Uslu (2006) and Hegab et al. (2016).
Results of erythrogram revealed significant macrocytic hypochromic anemia in the infected groups compared with Theileria negative group. The hemolytic anemia occurs due to presence of parasites inside the RBCs causes hemolysis result from erythrocytic fragility (Mahmoud et al., 2019) and stimulation of erythrophagocytosis in reticuloendothelial system (Razmi et al., 2019). A permanent tick blood sucking causing chronic blood loss (Minnat and Abdulwadood, 2012). These results agreed with the results recorded by Al-Fetly (2012), Razavi et al. (2015) and Sohail et al. (2018).
The leukogram of infected groups showed significant leukopenia due to neutropenia, lymphopenia, monocytopenia and eosinopenia, compared with the Theileria negative group. This leukopenia may be explained as a results of destruction of infected lymphocytes due to presence of schizogony of parasite inside them (Mahmoud et al., 2019) while the decrease of other cells may be due to the suppression of bone marrow and leukopoiesis as a result of parasitic products and increase TNF-α production (Razmi et al., 2019). Moreover, Weiss and Wardrop (2010) reported that the eosinopenia and pancytopenia in Theileria infected animals may be due to parasitic toxins or different causes of necrosis, fibrosis, or suppression in bone marrow. These results were in agreement with Çöl and Uslu, (2006), Yaghfoori et al. (2017), Minnat and Abdulwadood (2012) and Khan et al. (2017) while leukocytosis in Theileria infected sheep were demonstrated in some studies that results due to stage of infection and proliferation of lymphocytes in the lymphoid organs as a defensive response to parasitic infection (Al-Fetly, 2012; Sohail et al., 2018; Razmi et al., 2019).
Significant thrombocytopenia was also reported in the infected groups compared with Theileria negative group. The thrombocytopenia in Theileria infection occurred due to increased platelets destruction, consumption and degranulation in peripheral circulation (Çöl and Uslu, 2006) and/or due to the pancytopenia resulting from production of TNF-α or bone marrow suppression as result of parasitic toxins (Razmi et al., 2019).
The liver affection during ovine theileriosis was proved by significant hypoproteinemia, hypoalbuminemia, total and indirect hyperbilirubinemia. The AST and GGT activities were significant increased. Moreover, the values of A:G ratio and direct bilirubin have no significant changes in both infected groups compared with the Theileria negative group. Hypoproteinemia and hypoalbuminemia in Theileria infection might be resulted from synthesis reduction in liver due to liver failure, extravascular release proteinaceous fluids from diseased lymph nodes and/or reduction in protein intake due to anorexia during infection (Sohail et al., 2018; Mahmoud et al., 2019). The increased liver enzymes activities were explained by the leakage from liver damages as necrosis and degeneration of the hepatocytes, severe damage of the hepatobiliary system and increased sinusoidal spaces (Gunes et al., 2016; Akhter et al., 2017; Yaghfoori et al., 2017). The total and indirect hyperbilirubinemia were due to hemolysis of infected RBCs inside the reticuloendothelial system causing hemolytic anemia and liver failure (Akhter et al., 2017; Yaghfoori et al., 2017; Mahmoud et al., 2019).
The kidney function tests revealed significant increase of BUN and creatinine; azotemia of both infected groups compared with Theileria negative group. This azotemia may be related to the deposition of immune complexes of Theileria spp. in the renal tissue causing focal or diffuse coagulative necrosis and/or increased protein catabolism due to inappetence during Theileria infection (Baghshani et al., 2012; Elbasheir and Elhussein, 2013; Yaghfoori et al., 2017).
The histopathological changes included depletion and necrosis with hemorrhages of lymphoid follicles of lymph nodes and diffuse depletion of the lymphoid tissue with degenerative changes and hemorrhages in the spleen. The lymphocytic depletion and necrosis may explain the recorded lymphopenia in the Theileria infected groups (Akhter et al., 2017).
According to previous studies, a conserved gene of 18S rRNA is specific target for Theileria species detection by using PCR assay as described by Liu et al., 2010 as a sensitive diagnostic tool for ovine theileriosis. In this study, different primer sets were used for Theileria spp.; a universal primer (Allsopp et al., 1993) and species-specific primers for T. ovis (Altay et al., 2005) and T. lestoquardi (Kirvar et al., 1998). Different Theileria species as T. ovis and T. lestoquardi were identified by using specific primer sets. From positive samples of universal primers 36.8% were identified as 53.6% T. ovis and 46.4% mixed infection with T. ovis and T. leastoquardi.
Sequencing and phylogenetic analysis were performed using a blood sample collected from Al-Saff (Giza) that was positive to T. lestoquardi and T. ovis. The 18S rRNA gene of T. lestoquardi and T. ovis were partially sequenced (GenBank Accession Numbers MT002826 and MT002827). The isolate of T. leastoquardi (MT002826) revealed 100% homology with T. lestoquardi from bovine/Egypt (KF771185), ovine/Tunisia (KM117212 and KJ458988), ovine/China (AF081135) and ovine/Iraq (KC778786). This isolate showed 99% homology with T. annulata from France (KF429799), Turkey (AY508463), Pakistan (MG599090) and Spain (DQ287944). Our isolate was clustered in a single clade with T. lestoquardi, T. annulata, T. parva and T. taurotragi. This result is consistent with a previous report about T. lestoquardi from ovine source in Tunisia (Rjeibi et al., 2016) and T. lestoquardi from buffaloes source in Egypt (AL-Hosary et al., 2015). The isolate of T. ovis (MT002827) revealed 100% homology with T. ovis from South Africa (MG203885), France (EU622911), Turkey (KT851436), China (FJ603460), Tanzania (MG725961) and India (MH819508).
CONCLUSION
The infected sheep showed several clinical manifestations as pyrexia, cachexia, emaciation, lacrimation, conjunctivitis, pale mucous membrane and tick bite wounds. Hematological examination of the ovine theileriosis showed macrocytic hypochromic anemia with pancytopenia affecting the three lineages of hematopoiesis. Moreover, the disease causes multiple organ affections reflected in biochemical results as hypoproteinemia, hypoalbuminemia, hyperbilirubinemia, azotemia with increase of liver enzymes activities. according to our knowledge, this is the first report of phylogeny of T. lestoquardi in sheep in Egypt.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the veterinarians in veterinary clinics, sheep farms and slaughterhouse who helped in sample collection. We also thank the staff of Genome Research Unit and Clinical Pathology unit in Animal Health Research Institute (AHRI) and Department of Clinical Pathology, Faculty of Veterinary Medicine for supportive technical assistance.
Author’s Contribution
Mostafa Bashandy, Khaled Mahran and Waheed Mousa: Conceptualization.
Mahmoud Eliwa: Samples collection.
Mahmoud Eliwa and Khaled Mahran: Cinicopathologic investigations.
Mahmoud Eliwa and Naglaa Hagag: Molecular studies.
Mohamed Shaalan: Histopathological examination.
Khaled Mahran: Data analysis.
Mahmoud Eliwa and Khaled Mahran: Writing the manuscript.
All authors read and approved the final version.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES