Advances in Animal and Veterinary Sciences
Ectoparasite Fauna of Commensal Rodents Collected from the North Sinai Governorate - Egypt and its Public Health Significance
Doaa S. Farid1, Eman M. Abouelhassan2*, Ali A. El- Sebae1, Mohamed E. Enany3, Ahmed I. Youssef4
1Department of Environmental Protection, Faculty of Environmental Agricultural Sciences, Arish University, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Egypt; 3Department of Microbiology & Immunology (Bacteriology), Faculty of Veterinary Medicine, Suez Canal University, Egypt; 4Department of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, Suez Canal University, Egypt.
Abstract | Rodents have a huge impact on the natural environment. Rat and mice are considered a natural host for a large number of ectoparasites. The present study aimed to determine the geographical distribution and ectoparasites infestation rates of commensal rats and mice collected from the North Sinai governorate - Egypt. The survey was conducted during the period from December 2016 to November 2017 in three different locations namely Bir el-`Abd city, Rabaa, and Qatia villages located in the North Sinai governorate. The ectoparasites were isolated from four species of rodents including Rattus norvegicus (Brown rats), Rattus rattus frugivorus (Black rats), Rattus rattus alexandrinus, and Mus musculus (House mouse). The survey recovered and identified three species of fleas including Echidnophaga gallinacea, Xenopsylla cheopis, and Leptopsylla segnis, four species of lice including Hoplopleura hirsute, Hoplopleura ocanthopus, Hoplopleura oenomydis, Polyplax spinulosai, and five species of mites including Laelaps nuttalli, Dermanyssus gallinae, Ornithonyssus bacoti, Myobia musculi, Allodermanyssus sanguine. The distribution of the ectoparasites was identified according to several factors including rodent species, sex, location, and seasonal effects and the zoonotic role of some of the identified rodent ectoparasites was discussed.
Keywords | Rodents, Ectoparasite, North Sinai, Fleas, Egypt
Received | October 14, 2020; Accepted | December 12, 2020; Published | February 20, 2021
*Correspondence | Eman M. Abouelassan, Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Egypt; Email: [email protected]
Citation | Farid DS, Abouelhassan EM, El-Sebae AA, Enany ME, Youssef AI (2021). Ectoparasite fauna of commensal rodents collected from the north sinai governorate - egypt and its public health significance. Adv. Anim. Vet. Sci. 9(4): 563-570.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.563.570
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abouelhassan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Rodents have an important role in the maintenance and transmission of vector-borne zoonoses (Tomassone et al., 2018), they are considered the largest mammals order with wide species diversity; they can be found in all terrestrial environments which support life (Vaughan, et al., 2000). Rodents presented one of the health problems worldwide (Pakdad et al., 2012). They have huge negative impacts on the natural environment causing great economic losses to the livestock and human in direct and indirect ways (Harpera and Bunbury, 2015). Rodents can induce damage to properties like homes, apartments, hotels, office complexes, retail, and warehouse facilities, as well as, public utility operations (Abdel- Azeem, 2008). In urban environments, rodent species such as Norway rats (Rattus norvegicus) are prevalent and pose a threat to public health by serving as a vector and/or reservoir host with their ectoparasites for various pathogens that can be transmitted to humans and livestock (Chagas et al., 2017).
Ectoparasites that infest rodents include various species of fleas, lice (Insecta), ticks, and mites (Acari); they can serve as vectors of several diseases including bubonic plague, murine typhus, and tularemia (Mohd Zain et al., 2015).
From the public health perspective, rodents considered a key role in the transmission of many zoonotic diseases such as plague, leishmaniasis, typhus, and salmonellosis (Hamidi et al., 2015). Besides, they can host some of the ectoparasites that act as vectors of pathogens as bacteria, viruses, helminths, and protozoa with their health problems in human (Kia et al., 2009). Rodents can harbor four groups of ectoparasites fleas (Siphonaptera), ticks (Acari), mites (Mesostigmata), and lice (Siphonaptera) (Asiry and Fetoh 2014). Flea species are considered to be one of the most important vectors of human infectious diseases in the world, for instance, they are capable of transmitting plague through their bites, particularly Xenopsylla cheopis, additionally, they are playing a role in the transmission of Bartonella spp. and tularemia (Bendek et al., 2011). Concerning the rodent lice, they are important vectors for Haemobartonella muris and Rickettsia typhi (Farahat et al., 2014). Mites are temporally ectoparasites that can infest humans and animals, causing severe irritation by their bites, which may lead to allergic dermatitis or respiratory allergy especially in children (Farahat et al., 2014).
In Egypt, rats and mice are considered the most damaging wild vertebrates based on economic losses and health-related issues. They are very common in many Egyptian Governorates (Yassin, 2009). Consequently, the data on rodents and their relationships with ectoparasites in Egypt is limited, therefore, the main objective of this study was to determine the distribution and infestation rates of ectoparasites that parasitizing rats and mice in this area and discuss our results in the context of possible zoonotic disease risk based on the hosts and vectors present.
Materials and Methods
Rodent’s collection
The study was designed as cross-sectional study considering the detection of the period prevalence of the rate of infestation amonge rats in the North Sinai governorate- Egypt. The survey was conducted during the period from December 2016 to November 2017 in three geographical locations namely Bir el-`Abd city, Rabaa, and Qatia villages) located at the North Sinai governorate, Egypt. Rodent species were collected by applying the common wire traps.
The traps were placed close to the walls inside and outside houses and near the household poultry and livestock animals (sheep and goat). Before applying, the traps were thoroughly cleaned with hot water and quaternary ammonium compound based detergent and baited with fresh food (tomato slices, carrot, fish, cucumber, cheese, and canned salmon). The traps were distributed in the evening and checked in the early morning before sunrise. The baits were changed from time to time to ensure maximum catch probability.
The captured rodents were transported to the laboratory, Department of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, Suez Canal University.
Parasitological examination
Sampling: A total of 119 rodents (rats and mice species) were captured from the three different locations in North Sinai Governorate, Egypt, during the period from December 2016 to November 2017. The captured rodents were examined for ectoparasites by brushing their bodies starting from the head, backward to the neck, the trunk, and, the tail. The examination included ears, surrounding eyes, head, and other parts in the body. The fur of captured rodents was gently scraped off using a fine brush to detach the external parasites. Mites were quickly picked up using the toothbrush. The ectoparasite samples of each animal were fixed in 70% alcohol in separate specimen bottles referring to the date, area of collection, the species and, the sex of rodents.
Ectoparasites preparation
Preparation and identification of fleas and lice: The collected fleas and lice were prepared using clearing with 10% Potassium Hydroxide solution (KOH) 10%, followed by dehydration, mounting, and fixation with Canada. The mounted slides were examined by stereo-microscopic (BoEco, Germany- 2X). The ectoparasites species were identified based on the systematic identification keys of Soulsby, (1982).
Preparation and identification of mites: Mite species were mounted on glass slides by 70% alcohol after rinsing in water using Berlese medium (Hoyer´s) which is prepared according to Honey et al. (2014). Species identification of mites was carried out according to the available systematic identification key by (Hendrix, 2011).
Statistical analysis
Statistical analysis was carried out using statistical package for social sciences – SPSS version 24 (Green and Salkind, 2016). The recorded results were analyzed using a multifactorial two-tailed Analysis of Variance (ANOVA) as the test of significance. P-value was considered highly significant at p<0.01 and significant at <0.05.
RESULTS
Prevalence of ectoparasite infestations of collected rodents
The collected rodent species were identified as Rattus norvegicus, Rattus rattus, and Mus musculus, based on the description of rodent species of Egypt (Osbron and Helmy, 1980). A total of 807 individual ectoparasites were collected and identified, they were classified into three groups as shown in Table 1, Fleas (Insecta: Siphonaptera), Sucking lice (Insecta: Anoplura), and Mites (Acarina: mesostigmata).
Table 1: Taxonomic taxa of ectoparasites species grouped by families collected from commensal rodents from three location of the North Sinai, Egypt.
|
Fleas (Species = 3) |
Lice (Species = 6) |
Mites (Species = 5) |
|
Insecta:Siphonaptera Phylum: Arthropoda, Class: Insecta (Hexapoda), Order: Siphonaptera
Family: Pulicidae Xenopsylla cheopis (136, 59.13%) Echidnophaga gallinacea (92, 40%)
Family:Leptopsyllinae *Leptopsylla segnis (2, 0.87%)
|
Insecta:Anoplura Phylum: Arthropoda, Class: Insecta (Hexapoda), Order: Anoplura
Family: Hoplopleuridae Hoplopleura hirsuta (21, 24.14%), H. ocanthopus (6, 6.90%), H. oenomydis (5, 5.75%), Hoplopleura spp (39, 44.83%).
Family:Polyplacidae Polyplax spinulosa (16, 18.39%) |
Acarina:mesostigmata Phylum: Arthropoda, Class: Arachnida, Order: Acarina, Suborder: Mesostigmata
Family: Dermanyssidae Ornithonyssus bacoti (77,15.71%) Allodermanys sussanguine (1, 0.20%) Dermanyssus gallinae (7, 1.43%)
Family: Laelaptidae Laelaps nuttalli (404, 82.45%)
Suborder: Prostigmata Family: Myobiidae Myobia musculi (1, 0.20%) |
Table 2: Prevlance of ectoparasites infestation in rodents species captured from the three localities in North Sinai Governorate, Egypt during the period from December 2016 till November 2017
| Ecto-parasite | No. Examined rodents examined | No of rodent with ectoparsites | Prevalence (%) | No of Ecto-parasite Individual | Constituent ratio (C) | Index | Mean Abundance (MA) |
|
Flea
|
119 | 67 | 56.3 | 230 |
230/807 28.50 |
1.93 |
230/67 (3.43) |
|
Lice |
119 | 40 | 33.61 | 87 | 10.78 | 0.73 | (2.18) |
|
Mites |
119 | 87 | 73.11 | 490 | 60.72 | 4.11 | 5.63 |
|
Total |
807 |
100 |
4.16 |
Table 3: Prevalence of ectoparasite infestations according to the collected rodent species, the host sex, area of collection and seasons.
| Item | No. Of Examined rodents | Total Prevalence No. (%) | Flea | Lice | Mites | |
| No (%) | No (%) | No (%) | ||||
|
Rat Species |
R. norvegicus | 82 | 69 (84.15) | 45 (54.88) | 31 (37.80) |
58 (70.73) |
| R. r. frugivorus | 28 | 27 (96.43) | 17 (60.71) | 9 (32.14) |
25 (89.29) |
|
| R. r.alexandrinus | 6 | 5 (83,33) | 5 (83.33) | 0 |
4 (66.67) |
|
| M. musculus | 3 | 0 | 0 | 0 |
0 |
|
| Total | 119 | 101 (84.87) | 67 (56.30) | 40 (33.61) | 87 (73.11) | |
| Rodent sex | Male | 54 | 45 (83.33) | 33 (61.11) | 12 (22.22) |
36 (66.67) |
| Female | 65 | 56 (86.15) | 34 (52.31) | 28 (43.08) |
51 (78.46) |
|
| Locations | Bir el-`Abd | 76 | 70 (92.11) | 39 (51.32) | 25 (32.89) |
55 (72.37) |
| Rabaa | 23 | 23 (100) | 16 (69.57) | 8 (34.78) |
16 (69.57) |
|
| Qatia | 20 | 18 (90) | 12 (60) | 7(35) |
16 (80) |
|
| Seasons | Winter | 24 |
18 (75) |
1.33c±0.08 |
3.33a±0.03 |
3.66b±0.18 |
| Spring | 27 |
24 (88.89) |
7.0a±0.64 |
2.66a±0.03 |
8.00a±0.08 |
|
| Summer | 26 |
24 (92.31) |
4.66ab±0.06 |
3.00a±0.05 |
8.00a±0.15 |
|
| Autumn | 42 |
35 (83.33) |
9.33a±0.23 |
4.33a±0.66 |
9.33a±0.62 |
|
| P-value | 0.005 | 0.801 | 0.045 |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P<0.05).
SE=standard error.
Prevalence of ectoparasite infestations according to the rodent species
R. norvegicus, R.r.frugivorus were the most infested individuals, they were parasitized with three groups of ectoparasites; fleas, lice, and mites. On the other hand, and R.r. alexandrinus was parasitized with only two groups of ectoparasites; fleas and mites. M. musculus didn’t show any infestation with the ectoparasites species (Table 2).
Prevalence of ectoparasite infestations according to the location.
In the present study, there is a change in the prevalence rate with different geographical locations. The highest infestation rate was localized in Rabaa although the number of rodents collected from this area was the lowest. Concerning the mites and, lice, the highest infestation rate was detected in the Qatia by 80% and 35%, respectively, while the flea highest infestation rate was detected in Rabaa by 69.57% (Table 3).
Prevalence of ectoparasite infestations according to the host sex.
The ectoparasites’ infestation rate was higher in females than males, the infestation rate of male individuals was 83.33%, while in females was 86.15% (Table 3).
Prevalence of ectoparasite infestations according to the seasons.
The highest prevalence rates were recorded in the autumn season for both Flea and Mites, meanwhile, there were no significant differences detected in lice infestations with the seasonal fluctuations (Table 3).
Morphological description of the collected ectoparasites
Morphological description of the collected fleas (Insecta: Siphonaptera)
Family: Pulicidae
Xenopsylla cheopis (Rothschild,1903)
Angulated head, wingless insects compressed laterally, dark brown, the thorax is not reduced, the thoracic segments together are wider than the first abdominal segment, absence of ctenidia, more than one post occipital bristles, and the presence of mesopleural rod Figures 1 D and E.
Echidnophaga gallinacea (Westwood, 1875)
Angulated fronts, well-developed occipital lobe, genal lobe directed backward, the thorax narrower dorsally than the tergum of the first abdominal segment, absence of ctenidia, and the presence of two posts occipital bristle Figures 1 A and B.
Family:Leptopsyllinae
Leptopsylla segnis (Schönherr, 1811)
The presence of genal and pronotal ctenidia, vertical genal ctenidia composed of four elements, and pronotal ctenidia present as presented in Figure 1C.
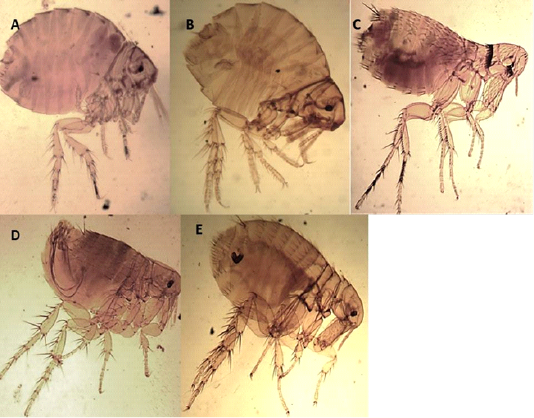
Figure 1: Light microscopy (LM) of permanent preparation of Flea spp. (X10) A&B: Echidnophaga gallinacea; C: Leptopsylla segnis; D& E: Xenopsylla cheopis
Morphological description of the collected sucking lice (Insecta: Anoplura)
Family: Hoplopleuridae (Ferris, 1951)
The Antennae were five-segmented, similar in males and females. The first pair of legs were small with slender claws, the second pair was larger with broader claws, and the paratergal plates project apically from the body with distinct tergal and sternal plates.
Hoplopleura hirsuta
Characterized by the presence of six pairs of distal abdominal setae, females presented paratergal plates by 4-6 only slightly elongated, and males with only seven tergal plates bearing a row of setae (Figures 2 A, B, C).
H. ocanthopus
Slender, 1-2 mm, characterized by large parategal plates which are four and five with two large setae on posterior margin (Figures 2 D, E, F).
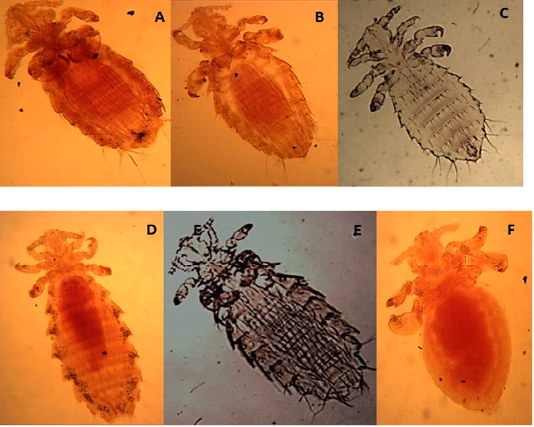
Figure 2: Light microscopy (LM) of permanent preparation of lice spp. (X40) A,B&C: Hoplopleura hirsuta; D, E& F: Hoplopleura ocanthopus
H. oenomydis
Abdomen with setae in the membrane between sternal and paratergal plates (Figures 3A and B).
Family:Polyplacidae
Polyplax spinulosa
Paratergal plates 3-5 with only dorsal apical angle produced into a point as in Figures 3C and D.
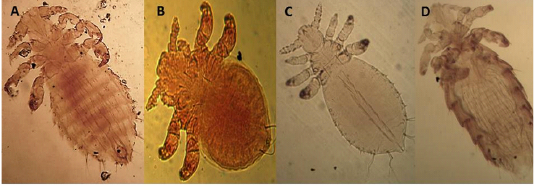
Figure 3: Light microscopy (LM) of permanent preparation of lice spp. (X40) A&B: Hoplopleura oenomydis; C& D: Polyplax spinulosa
Morphological description of the collected mites
Family: Dermanyssidae (Kolenta, 1859)
Ornithonyssus bacoti (Hirst,1931)
The dorsal plate is narrower than in the other species and tapers gradually to a blunt point Setae of the same size. There are no teeth on the chelicera (Figures 4A and B).
Dermanyssus gallinae (DeGeer, 1778)
The dorsal shield does not reach the post end and the posted margin. The setae are smaller on it than around the dorsal plate (Figures 5 A and B).
Allodermanyssus sanguine (Hirst, 1914)
Has 2 dorsal plates, the sternal plate bears all three pair of setae (Figure 4 D).
Family: Laelaptidae
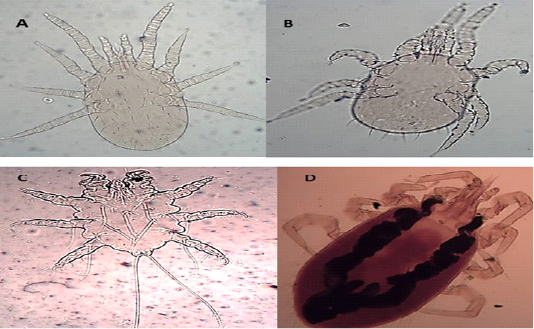
Figure 4: Light microscopy (LM) of permanent preparation of Mite spp. (X10) A&B: Ornithonyssus bacoti; C: Myobia muscula; D: Allodermanyssus sanguine
Laelaps nuttalli (Hirst, 1915)
Hypostome has a dorsal labrum of two lobes covered with minute papillae, segmented chelicerae, and ventral labium carrying two median lobes with laciniae and two lateral club-like lobes. Palpal and foreleg tarsal organs comprised ten and fifteen sensilla, respectively. Each pulvillus terminated with two medioventral claws and integumental folds beside longitudinal folds (Figures 5 C and D).
Family: Myobiidae
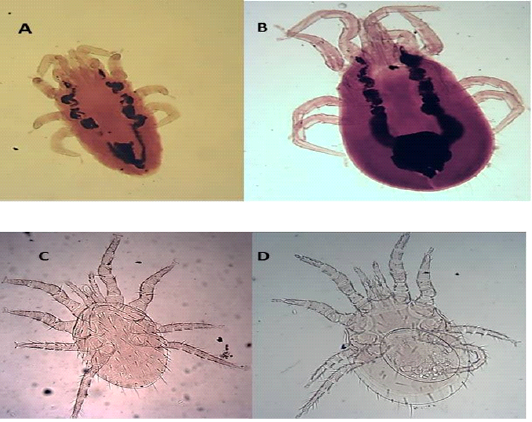
Figure 5: Light microscopy (LM) of permanent preparation of Mite spp. (X10) A&B: Dermanyssus gallinae; C& D: Laelaps nuttalli
Myobia musculi (Schranck, 1781)
has four pairs of legs, but the first pair is very short as an adaptation for grasping the hair shaft and giving the second pair of legs end in empodia (claw-like structures) (Figure 4 C).
Discussion
Rodents play an important role in the transmission of several pathogens, also, they affect the arthropod vectors abundance; consequently, the increase in zoonotic risk (Tomassone et al., 2018). One of these important rodents is rats that act as hosts for vector-borne diseases; they act as a source of infection for vectors besides they serve as hosts for the vectors themselves, supporting their populations (Easterbrook et al., 2007). They provide a stable habitat for a wide variety of ectoparasites that are considered as vectors of many zoonotic diseases (Krasnov et al., 2004) such as plague, leishmaniasis, and typhus. The risk of human exposure to these numerous pathogens is a public health concern, some of these pathogens can cause less or even non- symptomatic diseases in humans, besides, there are difficulties in the diagnosis of these infections in humans (Easterbrook et al., 2007). Several epidemiological studies described the relationship between the rodents and their vectors in a high prevalence and great diversity. For instance, the relationship in rodents and their flea parasites with Bartonella species are complicated, evolving scenarios and reflect the high degree of adaptation of these organisms (Gutiérrez et al., 2015).
The rodent species captured in this survey were R. norvegicus, R. r. frugivorus, R. r. alexandrinus, and M. musculus. They are the most common rat species of ecological and public health significance (Bonnefoy et al., 2008). These species were also recorded in Egypt by El Kady et al. (2008) in the Dakahlia governorate, Mmetwaly et al. (2009) in Behera governorate, Yassin, (2009) in Qaliubiya, and Soliman et al. (2010) in Menofiya.
The current findings revealed that the overall prevalence of infested rodents with ectoparasites was 84.87%, the highest ectoparasite infestation rates were recorded in Rabaa. Some studies also recorded the relationship between the density, prevalence of ectoparasite groups, and the localities of the hosts such as Farahat et al. (2014).
Mites and fleas were the most predominant ectoparasites of the all-identified rodents species, with exception of Mus musculs that didn’t show any infestation with the ectoparasites species. The high infestation rate of ectoparasite species in Rattus norvegicus and Rattus rattus were attributed to the ecological and behavioral characters related to each species, as well as, these species’ preferences for more crowded localities where houses and cultivated areas were located (Heaney et al., 2005). In addition to the morphological features such as the size and differences in the skin and the fur covering it (Hamidi et al., 2015), Rattus species are characterized by dense fur comparing with M. musculus which are small in size with thin fur (Solanki et al., 2013).
Fleas species can transmit many zoonotic pathogens from rodents to humans through their bites. Such as Yersinia pestis which is the causative agent of Plague. In the current study, three species of flea were identified E. gallinacea, X. Cheopis, and L. segnis. These species were also recorded in Egypt by Soliman and Mikhail, (2011), Desoky et al. (2014) and Loftis et al. (2006), who also recovered these species from mammals in 17 cities of Egypt.
The current study revealed that four species of lice were recovered; Hoplopleura hirsuta, Hoplopleura ocanthopus, Hoplopleura oenomydis, and Polyplax spinulosa. The present findings went parallel with those reported by Abdel-Azeem, (2008), Pakdad et al. (2012) and Chakma et al. (2017) who recorded the same species infesting rodents. Lice are species-specific (Madinah, et al., 2013) and they harbor zoonotic pathogens such as plague bacilli and can transmit tularemia and bartonellosis to humans, besides, their bites can cause severe pain, irritation, and anemia in severe infestation (Bowman et al., 2003).
Mites are temporally blood-sucking ectoparasites of mammals. Rodent mites frequently attack humans living in rodent-infested buildings and their bites may produce irritation and sometimes painful allergic dermatitis or mite respiratory allergy particularly in children (Bakr et al., 1995). Mite species could play a role in the epidemiological triad of murine typhus and the Seoul virus (Himsworth et al., 2015). The current study recovered five species of mites that were identified as Laelaps nuttalli, Dermanyssus gallinae, Ornithonyssus bacoti, Myobia musculi, and Allodermanyssus sanguine. Many studies reported these mites species on different hosts including Bahgat (2013), Soliman et al. (2001) and Telmadarraiy et al. (2007).
the present study revealed the presence of a significant difference between the ectoparasites infestation and the seasonal variations concerning flea and mites. The severity of infestation was observed during autumn, while the lowest infestation was recorded in winter as the climatological and other macroclimatic environmental conditions greatly influenced rodent hosts, as well as, the survival and multiplication of their ectoparasites (Hamidi et al., 2015). These results were in agreement with those recorded by Bakr et al. (1995) in Egypt. The reduction of the prevalence rate of flea during winter months may be due to the depression in flea larvae multiplication and activity in such weather. On the other hand, there is no significant difference between the lice infestation and the season’s changes was recorded.
The ectoparasites infestation rate was higher in females than males due to physiological factors as the differences in blood hormonal levels, besides the alterations of the hormonal levels as a response to stresses when females repeatedly exit the nests to gain more food during their pregnancy and lactating, similar findings were recorded by Kia et al. (2009) and Asiry and Fetoh (2014).
Conclusion
The study was carried out to establish baseline data for ectoparasite-infested rodents in the North Sinai region - Egypt and may help for appropriate planning to control and eradicate zoonotic diseases in this area. Further studies are required to determine new methods of control for these rodents and ectoparasites.
Acknowledgment
Sincere thanking to Prof. Awad Farahat El-Bahrawy for his generous directions with the ectoparasite identification.
Ethics
This study was approved by the Ethics Committee of the Suez Canal University. All animal experiments were conducted following the guidelines of the Guide for the Care and Use of Laboratory Animals, Faculty of Veterinary Medicine Science, Suez Canal University, Egypt (approval number 2020004).
Conflict of interest
The authors have no conflicts of interest relevant to this article.
authors contribution
All the authors contributed equally during the study.
References






