Advances in Animal and Veterinary Sciences
Research Article
Comparative Study Between the Post Vaccinal Histopathological Alterations in Some Broiler Chickens’ Tissues Induced by Lasota and VG/GA Newcastle Disease Vaccinal Strains
Manar A. Khader1, Magdy F. El-Kady2, Iman B. Shaheed3*
1Department of Pathology, Faculty of Veterinary Medicine, Al Azhar, University, Gaza, Palestine; 2Department of Poultry Diseases, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt; 3Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Abstract | The control of Newcastle disease virus (NDV) depends on vaccination to protect chickens against clinical signs and mortalities. Live vaccines are usually used due to it slow cost and easy application. This study was performed to evaluate post-vaccinal reactions of LaSota and VG/GA (AVINEW®) vaccines in broiler chickens. One hundred twenty day- old commercial broiler chicks were divided into three groups (40 each). Group 1 maintained as non-vaccinated group (negative control), Group 2 was vaccinated with LaSota vaccine while Group 3 was vaccinated with AVINEW® vaccine by ocular rout at day 10 of age. All chicks were vaccinated with infectious bursal disease vaccine at 12th day. Blood samples were collected weekly from each group. Three chicks were sacrificed from each group at 2-, 5-, 10- and 15-days post vaccination (dpv). Tissue samples were collected from trachea, kidneys, proventriculus, small intestine, spleen and Harderian gland for histopathological examination. Neither clinical signs nor mortalities were recorded in vaccinated groups. Petechial hemorrhage in Harderian gland was detected in both vaccinated groups. No significant difference in antibody titers was observed between both vaccinated groups. Histologically, edema, inflammatory cells infiltration, and focal deciliation were detected in trachea of both vaccinated groups. Avinew® vaccine produced more potent post-vaccinal reaction than LaSota in kidneys and small intestine. Meanwhile reactions on trachea, proventriculus, spleen and Harderian gland were almost identical.
Keywords | Histopathology, LaSota, NDV, Spleen, Trachea
Received | March 30, 2021; Accepted | April 10, 2021; Published | July 01, 2021
*Correspondence | Iman B Shaheed, Department of Pathology and Acting Dean of Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; Email: [email protected]
Citation | Khader MA, El-Kady MF, Shaheed IB (2021). Comparative study between the post vaccinal histopathological alterations in some broiler chickens’ tissues induced by lasota and vg/ga newcastle disease vaccinal strains. Adv. Anim. Vet. Sci. 9(8): 1135-1142.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1135.1142
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Soliman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Newcastle disease (ND) is one of the most critical avian diseases affecting poultry production (Mousa et al., 2019). ND is caused by Avian orthoavulavirus-1 which has a negative sense single-stranded non segmented RNA genome (Bello et al., 2019).
According to the phylogenetic analysis, intravenous pathogenicity index (IVPI), intracerebral pathogenicity index (ICPI), and mean death time (MDT) NDV strains are grouped as highly virulent (velogenic), moderately virulent (mesogenic) and avirulent (lentogenic) (Sedeik et al., 2019). NDV affects about 240 species of birds. Infection occurs by virus inhalation, ingestion or through conjunctiva (Mariappan et al., 2018). The clinical signs of ND depend on the virus strain, host species, environmental stress, co-infection with other microorganisms, and host immune status. ND is characterized by respiratory, nervous, and enteric infections (Gowthaman et al., 2019).
Live and inactivated NDV vaccines are commonly used to control ND across the world (Bello et al., 2019; Manar et al., 2020). Live ND vaccines stimulate systemic and mucosal immune responses similar to natural infection, because of their ability to replicate in chicken tissues regardless the site of administration (Bello et al., 2019). Additionally, they are suitable for mass application via drinking water or spray (Roohani et al., 2015). Immunity is achieved by neutralizing antibodies formed against the viral hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins, which are responsible for virus attachment and spread (Kapczynski, et al., 2013).
Lentogenic NDV strains such as B1, F, LaSota, V4, VG/GA and I2 are used as live vaccines for ND control. LaSota is the most widely used strain because of its maximum immunogenicity. B1-based live ND vaccines are highly attenuated with no post-vaccinal reactions in birds. V4 and I2 vaccines are relatively thermostable (Senne et al., 2004). VG/AG (AVINEW®) vaccine is mainly enteric viscerotropic (Miller, 2008).
Experimental infections of specific pathogen-free (SPF) chickens with NDV vaccine strains lead to little to no clinical disease. However, the application of the vaccine under field conditions can decrease the flock productivity by causing mild respiratory symptoms, which could be increased in the presence of other respiratory pathogens or in combination with environmental stressors (Ellakany et al., 2018).
The objective of this study was to compare the post-vaccinal pathological changes between LaSota and VG/AG (AVINEW®) ND vaccines in broilers.
Materials and methods
Ethical committee approval
The protocol of this study was approved by Institutional Animal Care and Use Committee (IACUC), Cairo University, Egypt (Approval number, CU/II/F/101/18).
Vaccines used in the experiment
Commercial LaSota vaccine: Lyophilized vaccine consists of a live, lentogenic NDV, 1000 dose each dose consists of 106 EID50 of vaccinal virus. It manufactured by Genera Company– Croatia.
Commercial AVINEW vaccine (AVINEW®): lyophilized vaccine consists of a live NDV VG/GA 2000 dose.
Infectious bursal disease vaccine: lyophilized Gumboro Intermediate vaccine VMG 91 strain, (Genera Company – Croatia) 1000 dose every dose contains 104 TCID50.
Experimental Design
One handerd twenty day old commercial broiler chicks obtained from Al-Ahram Poultry Company were used in our experiment. Chicks were raised hygienically in cleaned and disinfected experimental separate chambers in the Department of Poultry Diseases, Faculty of Veterinary Medicine, Beni-Suef University. Chicks were provided with feed and water ad libitum. Chicks were divided into three groups (40 each). Group 1 maintained as non-vaccinated group (negative control), Group 2 vaccinated with LaSota vaccine while Group 3 vaccinated with AVINEW® vaccine. Chicks vaccinated with LaSota and AVINEW@ vaccines via ocular route at day 10. All chicks were vaccinated against infectious bursal disease at day 12.
Sampling
Three chicks from each group were sacrificed at 2-, 5-, 10- and 15-dpv. Tissue specimens were collected from trachea, spleen, Harderian gland, kidneys, proventriculus, and small intestine for histopathological examination. Blood samples were taken from eight chicks in each group weekly for Haemagglutination inhibition (HI) test.
Postmortem and histopathological examination
Clinical signs and postmortem lesions were monitored all over the time of the study. Tissue specimens were fixed in 10% neutral buffered formalin and routinely processed. Tissue sections were stained by H&E (Suvarna et al., 2013). Sections were examined using Olympus light microscope and captured by Optika camera using Optika vision pro software.
Scoring system for infected tissues
Lesion scoring for the collected tissues were done according to Hussein et al. (2018) and is shown in Table (1). Five random optical areas were inspected and figured then the mean of the five fields was recorded. Mean for 3 tissues ± standard error (SEM) was noted. Normal histological structure in all organs was given grade 0.
Haemagglutination inhibition (HI) test
Serum samples were collected from all groups for HI test which was carried out according to OIE, 2012 recommendations using 0.75% freshly prepared chickens RBCs suspension.
Table 1: Lesions scoring of NDV infected tissues according to Hussein et al., 2018.
| Organ | Grade | Lesions |
| Trachea | 1 | Inflammatory cells infiltration and hyperemia |
| 2 | Hyperemia, edema and inflammatory cells infiltration | |
| 3 | Hyperemia, inflammatory cells infiltration, edema and deciliation | |
| 4 | Mild hyperplasia and deciliation | |
| 5 | Hemorrhage, hyperplasia and desquamation. | |
| Kidneys | 1 | Mild accumulation of inflammatory cells in renal interstitium |
| 2 | Inflammatory cells in association with foci of degeneration in the renal tubules | |
| 3 | Inflammatory cells in association with foci of degeneration and necrosis in the tubules | |
| 4 | Inflammatory cells beside degeneration and necrosis of the epithelium of medullary tubules and renal glomeruopathy | |
| 5 | Hyaline casts accompanied by interstitial nephritis. | |
| Proventriculus | 1 | Mild epithelial cell degeneration and necrosis with heterophils |
| 2 | Extensive epithelial cell degeneration and necrosis accompanied by mononuclear cell infiltration | |
| 3 | Destruction of the lymphoid areas and often fibrin was present | |
| 4 | Destroyed lymphoid areas with some haemorrhage | |
|
Small intestin |
1 | Mild to moderate infiltration of lymphocytic cells |
| 2 | Moderate degeneration of the epithelial cell beside infiltration of mononuclear inflammatory cells usually in submucosal areas | |
| 3 | Haemorrhagic foci accompanied by necrosis in the mucosal lymphoid tissue | |
| Spleen | 1 | Mild hyperplasia or hypertrophy in the ellipsoids. |
| 2 | Proliferative lymphoid follicles | |
| 3 | Mild degeneration focally and lymphoid follicles in an active form | |
| 4 | Necrosis spread in focal manner and lymphoid follicles that were moderately active | |
| 5 | Diffuse necrosis and lymphoid follicles in a very active state | |
|
Harderian gland |
1 | Slight lymphocyte infiltration |
| 2 | Heavy lymphocyte infiltration | |
| 3 | Few lymphoid follicles formation | |
| 4 |
Much lymphoid follicles formation |
Statistical analysis
The statistical analyses were carried out using One-way factorial analysis of variance (ANOVA) was implemented to statistically analyze study results. Statistical significance was defined as (P≤0.05) using SPSS 25.
RESULTS
Clinical signs and postmortem lesions
Neither clinical signs nor mortalities were noticed in all groups all over the experiment. The only postmortem lesions detected were petechial hemorrhage in Harderian gland at 2, 5 and 10 dpv in both vaccinated groups.
Histopathology
No histological alterations were detected in all examined organs from group1 chicken (control group).
Trachea
Tracheal sections of vaccinated groups showed hyperplasia in goblet cells, edema and inflammatory cells infiltration in lamina propria at 2, 5 and 10dpv (Fig 1a). Focal areas of deciliation were noticed in vaccinated groups at 10dpv. Group 3 was characterized by presence of mucous at surface epithelium at 2 dpv (Fig 1b). Groups 2 and 3 showed increase in lymphocyte infiltration 15 dpv (Fig 1c). Significant differences in the histological alterations score were evident between vaccinated and unvaccinated group at 2, 5 and 10dpv. On the other hand, no significant differences were detected between vaccinated groups at all sacrifices.
Kidneys
Kidney sections of groups 2 and 3 showed hemorrhages and mild interstitial edema accompanied with inflammatory cells infiltration at 2dpv (Fig 1d). Group 3 showed foci of degeneration in renal tubules and inflammatory cells infiltrations at 2 and 5 dpv (Fig 1e and 1f). Group 2 lesions were significantly lower in severity compared with group 3 at 2, 5 and 10 dpv. Kidneys of group 3 were characterized by heavy infiltration with lymphocytes than group 2 at 10dpv (Fig 1g). No significant differences were seen between vaccinated and unvaccinated groups 15 dpv.
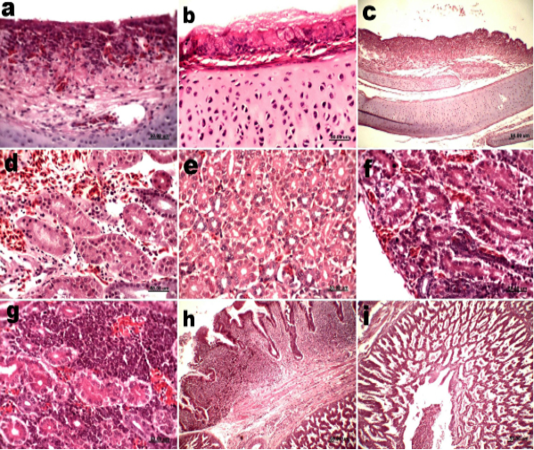
Figure 1: Histological sections of trachea, kidneys and proventriculus of vaccinated groups (a) Trachea group 2 at 5dpv showing inflammatory cells infiltration in lamina propria. (b) Trachea group 3 at 2dpv showing mucous in surface epithelium. (c) Trachea group 3 at 15dpv showing lymphocyte infiltration. (d) Kidneys group 2 at 2dpv showing mild edema in interstitial tissue and hemorrhage. (e) Kidneys group 3 at 2dpv showing foci of degeneration in renal tubules. (f) Kidneys group 3 at 5dpv showing degeneration in renal tubules and inflammatory cells infiltrations. (g) Kidneys group 3 at 10dpv showing heavy infiltration with lymphocytes. (h) Proventriculus group 2 at 5dpv showing mononuclear inflammatory cells infiltrations. (i) Proventriculus group 3 at 5dpv showing increased gland secretions which accumulated in the lumen.
Proventriculus
Proventricular sections of groups 2 and 3 showed mild epithelial cell degeneration and necrosis with mononuclear inflammatory cells infiltrations at 5dpv (Fig 1h). Both vaccinated Groups showed increased glandular secretions which accumulated in the lumen (Fig 1i). No significant difference in histological lesion score was detected between groups 2 and 3. Proventricular lesion scores 5dpv were significantly different between vaccinated and unvaccinated groups.
Small intestine
Groups 2 and 3 showed hyperplasia in intestinal glands 2 dpv (fig 2a). Group 3 showed heavy infiltration with mononuclear inflammatory cells infiltration compared with group 2 at 5dpv. Group 3 showed infiltration with lymphocytes at 10dpv (fig 2b). Only lymphocyte infiltrations were shown in both groups 15 dpv with the same severity (fig 2c). The only significant difference between groups 2 and 3 was recorded at 5dpv.
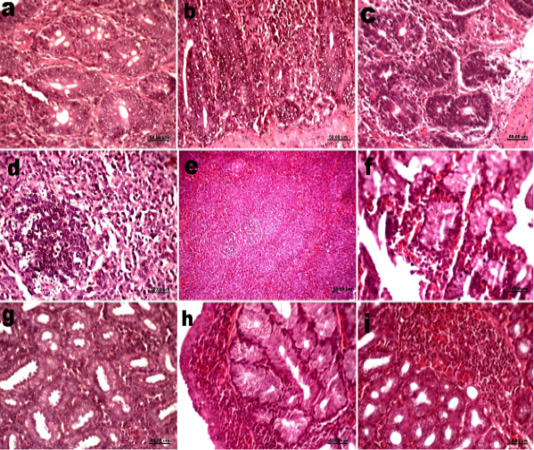
Figure 2: Histological sections of small intestine, spleen and Harderian gland of vaccinated groups (a) Small intestine groups 2 at 2dpv showing hyperplasia in intestinal glands. (b) Small intestine groups 3 at 10dpv showing infiltration with lymphocytes. (c) Small intestine groups 2 at 15dpv showing lymphocyte infiltrations. (d) Spleen groups 2 at 2dpv showing proliferation in lymphoid follicles. (e) Spleen groups 2 at 10dpv showing Activation of lymphoid follicles. (f) Harderian gland groups 2 at 2dpv showing hemorrhage. (g) Harderian gland groups 3 at 5dpv showing lymphocytes infiltrations. (h) Harderian gland groups 2 at 10dpv showing lymphoid follicles. (i) Harderian gland groups 3 at 15dpv showing lymphoid follicles.
Spleen
Spleens of vaccinated groups showed proliferation in lymphoid follicles (fig. 2d) and thickening of blood vessels wall 2- and 5 dpv. While at 10 dpv they showed increasing in bursal dependent lymphoid follicles (fig. 2e). No significance differences were detected in the histopathological alterations between groups 2 and 3 at all sacrifices. In the same time the histopathological lesion scores of vaccinated groups were significantly higher than the unvaccinated one at 2, 5 and 10 dpv.
Harderian gland
Groups 2 and 3 showed hemorrhage at 2 and 5 dpv (fig. 2f). Lymphocytes infiltrations in Harderian gland of groups 2 and 3 was mild at 2dpv and increased at 5dpv (fig. 2g). At 10 dpv lymphoid follicles appeared and continued till 15dpv in vaccinated groups (fig 2h, i). No significant differences were detected between groups 2 and 3 in all sacrifices.
Table 2: Histopathological lesions score induced by ND vaccines in trachea and kidneys at 2, 5, 10 and 15 dpv.
| Trachea | Kidneys | |||||||
| Gr | 2dpv | 5dpv | 10dpv | 15dpv | 2dpv | 5dpv | 10dpv | 15dpv |
| 1 |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
| 2 |
2.0± 0.45b |
2.60± 0.50b |
3.2± 0.37b |
0.40± 0.24a |
0.40± 0.24a |
0.40± 0.24a |
0.40± 0.24a |
0.20± 0.20a |
| 3 |
1.80± 0.37b |
1.80± 0.58b |
2.80± 0.37b |
0.40± 0.24a |
1.20± 0.37b |
1.00± 0.32b |
1.40± 0.24b |
0.20± 0.20a |
Values expressed as means ± SE; Different superscripts a, b, c and d indicate significant difference between values within the same row in the same scarification time.Significant values at P≤0.05.
Table 3: Histopathological lesions score induced by ND vaccines in proventriculus and small intestine at 2, 5, 10 and 15 dpv.
| Proventriculus | Small intestine | |||||||
| Gr | 2dpv | 5dpv | 10dpv | 15dpv | 2dpv | 5dpv | 10dpv | 15dpv |
| 1 |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
| 2 |
0.40± 0.55a |
1.00± 0.32b |
0.60± 0.24a |
0.20± 0.20a |
0.40± 0.24a,b |
0.60± 0.24a |
1.40± 0.24b |
0.80± 0.20b |
| 3 |
0.60± 0.55a |
1.40± 0.24b |
0.60± 0.24a |
0.20± 0.20a |
1.00± 0.32b |
1.40± 0.24b |
1.40± 0.24b |
0.80± 0.20b |
Values expressed as means ± SE; Different superscripts a, b, c and d indicate significant difference between values within the same row in the same scarification time.Significant values at P≤0.05.
Table 4: Histopathological lesions score induced by ND vaccines in spleen and Harderian gland at 2, 5, 10 and 15 dpv.
| Spleen | Harderian gland | |||||||
| Gr | 2dpv | 5dpv | 10dpv | 15dpv | 2dpv | 5dpv | 10dpv | 15dpv |
| 1 |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
0.00± 0.00a |
| 2 |
1.80± 0.37b |
1.20± 0.37b |
1.00± 0.32b |
0.40± 0.24a |
0.20± 0.20a |
1.40± 0.24b |
2.60± 0.24b |
2.60± 0.24b |
| 3 |
2.20± 0.37b |
1.20± 0.37b |
0.80± 0.20b |
0.40± 0.24a |
0.40± 0.24a |
1.20± 0.37b |
2.40± 0.24b |
2.60± 0.24b |
Values expressed as means ± SE; Different superscripts a, b, c and d indicate significant difference between values within the same row in the same scarification time.Significant values at P≤0.05.
The histopathological lesions scoring induced by ND vaccines in different organs were shown in Tables (2, 3, 4).
Table 5: Antibody titers against NDV in all groups.
| Gr. | 1 day |
1st week |
2nd week |
3rd week |
| 1 | 6.00±0.46 | 2.88±0.23 |
2.00±0.19a |
0.50±0.19a |
| 2 | 6.00±0.46 | 2.88±0.23 |
2.34±0.32a |
3.63±0.18b |
| 3 | 6.00±0.46 | 2.88±0.23 |
2.25±0.16a |
3.25±0.53b |
Haemagglutination inhibition (HI) test
Antibody titers against NDV were summarized in Table (5). No significant difference in antibody titers was noticed between all groups at 2nd week of age. However the antibody titers increased significantly at 3rd week age in groups 2 and 3 compared to group 1 with no significant difference between groups 2 and 3.
Discussion
ND is a major threat to poultry industry, causing high mortalities, reduction in growth and egg production (Cornax et al., 2012). Vaccination against ND is routinely performed all over the world to decrease losses from NDV infections (Senne et al., 2004). Vaccination against ND is achieved by either live or inactivated vaccines. The vaccine of choice should produce adequate immune response with minimal respiratory reactions (Perozo et al., 2008). Live ND vaccines produce systemic humoral immune response measured by antibodies level in serum (Erf, 2004). Additionally, the mucosal immunity represented by production of immunoglobulin A plays an important role in the development of protection against NDV (Scott, 2004). Antibody production in the mucosa is related to viral replication in the target cells; therefore the pathogenesis and tissue tropism of the viruses used for vaccination should be considered in order to evaluate the efficacy of a given live vaccine (Perozo et al., 2012). The efficacy of a live vaccine is correlated to its potency to multiply within the chicken and induce a satisfactory immune response (Abdul Ahad, 2012).
In this study we compared the pathogenesis of LaSota and AVINEW® ND vaccines in commercial chicks. The time of vaccination was achieved at day 10 to ensure the waning of maternal antibodies and to avoid interference with the active immunization. Vaccination was done by ocular route as it is the most efficient method to induce the highest antibody titer compared with drinking water and spray methods (Degefa T., et al. 2004; Elbayoumi et al., 2018).
HI antibody titer is a parameter that correlates to the protection induced by ND vaccines. At 2- and 3-weeks-old, HI titers were significantly higher in vaccinated groups than unvaccinated group. These results agree with Al-Shahery et al. (2008). At the same time LaSota vaccine produced antibodies titer higher than AVINEW® vaccine at 2- and 3-weeks-old but this increase was not significant. These findings agrees with Perozo et al. 2008 and Abdul Ahad, 2012. Who found no significant difference in values of antibody titre between the groups vaccinated with different vaccines.
In this study neither clinical signs nor mortalities were detected after vaccination. Additionally, the only postmortem lesion detected was the petechial hemorrhage in Harderian gland in both vaccinated groups. These results partially resemble Miller, (2008) who record mild conjunctivitis in some of vaccinated birds which resolved by 48 hours. Contrarily, the post vaccinal reaction in field conditions mentioned by Landman et al. 2017 were characterized by respiratory signs, increased mortality, and susceptibility to secondary bacterial infections mainly Escherichia coli.. This due to the course of the colibacillosis susceptibility of the broilers following ND vaccination might be dose-dependent too, resulting in higher susceptibility at seven days after vaccination using lower vaccine virus doses
Trachea of vaccinated groups showed edema and inflammatory cells infiltration, mucous in surface epithelium, blood vessels congestion and focal deciliation which come in the same line with Abdul Ahad, et al. (2012). The detected deciliation in vaccinated groups could be related to that simple squamous to cuboidal epithelium replaced the original pseudostratified epithelium (Mast et al., 2005). Fifteen days post vaccination, the epithelial damage was restored. The activation of the goblet cells observed in the vaccinated groups could indicate the initial NDV infection of these cells (Mast et al., 2005).
Group 2 showed non-significant increase in histopathological lesions score of trachea and antibodies titre than group 3. The same findings were recorded by Moghaddam et al. (2007) who mentioned that the increased histopathological lesions in the trachea of the vaccinated groups may assist to increased immune response.
No significant differences were detected in the histopathological changes of spleens of vaccinated groups. In the same time, the histopathological lesions of spleens of vaccinated groups were significantly higher than the unvaccinated one. The lymphocytic hyperplasia observed in the spleens in both vaccinated groups is in agreement with Mast et al. (2005) and Abdul Ahad, et al. (2012) and it could be attributed to the immune response induced by the vaccines.
Kidneys of vaccinated groups showed interstitial edema, degeneration in renal tubules and inflammatory cells infiltrations. AVINEW® vaccinated chickens showed significant increase in histopathological changes compared with groups 1 and 2 at 2, 5 and 10dpv.
No significant differences in pathological lesions score were detected between both vaccinated groups in proventriculus and Harderian gland at all sacrifices. The lesions recorded in Harderian gland continued till 15dpv could be related to the ocular rout of vaccination. The only significant difference in pathological lesion scores between both vaccinated groups was recorded at 5dpv which confirms that AVINEW® vaccine is of higher intestinal tropism (Perozo et al., 2008).
The histopathological lesions in our study were due to the vaccine virus multiplication which agrees with El Tayeb and Hanson (2002) who found that low virulence ND viruses can replicate only in areas where trypsin-like enzymes are present as in respiratory and intestinal tracts causing transient epithelial damage.
Acknowledgments
The authors are satisfied to grant the department of pathology, Cairo University and the department of poultry diseases, Beni-Suef University.
Conclusion
Histopathological changes and HI results showed that both LaSata and AVINEW® vaccines produce immunity against NDV which was clarified by antibody production. Additionally, both vaccines produced post vaccinal reactions which could facilitate the secondary infections in field conditions. AVINEW® vaccine produced more post vaccinal reaction than LaSota in kidneys and small intestine while it was equal to each other in trachea, proventriculus, spleen and Harderian gland.
Author’s contribution
MFE, and IBS designed the study. MAAK performed the experiments. MFK, IBS and MAAK, analyzed the data. MAAK, and IBS prepared the manuscript. All authors critically revised the manuscript for important intellectual contents and approved the final version.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES






