Advances in Animal and Veterinary Sciences
Comparison of Back and Loin Locomotor Bony Structures in Cat (Felis domestica) and Rabbit (Oryctolagus cuniculus): (Thoracic and Lumbar Vertebrae)
Sherif Khayri Abdelmoati Mohamed1,2*, Enas El-Hady1
1Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt; 2Faculty of Veterinary Medicine, Basic Veterinary Sciences, Hokkaido University, Sapporo 060-0818, Japan.
Abstract | This study investigated the comparative bony features of the thoracic and lumbar vertebrae of cat and rabbit. It was carried out on adult apparently healthy New Zealand twenty rabbits (Oryctolagus cuniculus) (two groups, 10 for each) and ten domestic cats (Felis domestica) of both sexes. Three-dimensional Computed Tomography was performed on the back and loin regions after anesthesia of both species using multi-slices CT system, followed by preparation of thoracic and lumbar vertebrae. Different measurements were taken and statistically analyzed for the vertebral structures of both species. The number of thoracic (T) and lumbar (L) vertebrae was 13/7 in cat while in rabbit was 13T/7L in 65% and 12T/7L in 35% of the animals studied. The rabbit had no anticlinal vertebra conversely in cat it was the (11th). There were collateral characteristic crests on the upper half of the first four thoracic spines in cat only. The lumbar vertebrae of rabbit had clear pointed hypapophysis in the first three bodies. In general, cat has longer bony measurements unless transverse lumbar process and lumbosacral space were notably increase in rabbit. The interlocking interarcuate articulation at the end of thoracic (11-13) and all lumbar vertebrae maximize the up and down movement but minimize the lateral movement that assist in forward speed of the cat. Although cat and rabbit are mammals, but both had different nutritional demands that might need more pace to pounce on the prey in cat rather than the rabbit whose fed on plants, so the aforementioned species-specific differences of thoracic and lumbar vertebrae in cat and rabbit affected greatly on their movement and locomotion.
Keywords | Cat, Computed Tomography, Locomotion, Rabbit, Vertebrae
Received | July 30, 2019; Accepted | August 24, 2019; Published | September 11, 2019
*Correspondence | Sherif Khayri Abdelmoati Mohamed, Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt; Email: [email protected]
Citation | Mohamed SKA, El-Hady E (2019). Comparison of back and loin locomotor bony structures in cat (felis domestica) and rabbit (oryctolagus cuniculus): (thoracic and lumbar vertebrae). Adv. Anim. Vet. Sci. 7(10): 819-828.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.10.819.828
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Mohammed and El-Hady. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The awareness of anatomical variations is significantly needed for surgical and radiological procedures in human and veterinary medicine. Thus, its importance for experimental research and surgical practice in laboratory and domestic animals (Swindle et al., 1988; Krotscheck et al., 2007).
Cats represent the most supremely efficient muscular machines in their ability to jump, twist and turn. The ratio of their strength to their size is far superior to humans. Domestic cat (Felis catus), a common buddy animal, is only one of the species in the family Felidae (Pitakarnnop et al., 2017).
Rabbit is economically efficient for numerous purposes as a source of meat and fur, high fertility, short gestation period, small size, low price and it is being used for antibodies production (Okerman, 1994). The number of cats and rabbits kept as pets in households has been increasing over the last years and they are often used as perfect laboratory animals in biomedical research tests as human substitutes and so, both of which require excellent knowledge of their anatomy (Norris Reinero et al., 2004; Abidu-Figueiredo et al. 2008; Xi et al., 2016).
Nowadays, Computed Tomography (C.T.) plays an important role in identifying the anatomical structures, diagnosis and evaluation of many human and veterinary diseases. Also, it used in many biometric researches to evaluate breeds (Regedon et al., 1991; Robina et al., 1991; Onar et al., 2002) but relatively few papers on normal C.T. anatomy in cat and rabbit are available.
Regarding the author knowledge, very limited data is available regarding axial skeleton of cat and rabbit anatomy in literature. Cat and Rabbit had different nutritional demands that might need more pace to pounce on the prey in cat rather than the rabbit whose fed on plants. Both species have the ability to trot and increase their velocity that is due to their integral locomotor system which capable them for getting their foods. Not only limbs but also the back and lion bony properties are involved in this system, so this study explore the comparison between thoracic and lumbar bony structures and their articular surfaces through several photomacrographs and C.T images in details that conflict their quick movement.
The current study is aimed to 1) elucidate the comparative anatomical features of the thoracic and lumbar vertebrae of cat and rabbit as educational tools in veterinary studies; 2) identify anatomical variations of C.T. images of the cat and rabbit thoracolumbar region for use by veterinary anatomists and radiologists; 3) give morphological aid and support for experimental research, clinicians and surgeons especially for determining the site of injectable anesthesia through lumbosacral space and 4) differentiate between the cat and rabbit carcasses by an anatomical bony illustration of the most differential points of their vertebrae to keep away from the commercial fraud in our country.
MATERIALS AND METHODS
Animals
Adult apparently healthy New Zealand rabbits (n = 20) and domestic cats (n = 10) of both sexes weighed about 2-4 kg were used. 20 months old rabbits were obtained from a laboratory farm in Faculty of Agriculture, Zagazig University. Rabbits were divided into two groups (1 and 2) according to the number of their thoracic vertebrae (n = 10 for each). The cats (10 months old) were purchased from a pet animal’s clinic in Zagazig city, Sharkia Governorate, Egypt. The live weight of animals was obtained by using a digital scale.
Sedation and Anesthesia
Both animals were injected 3 mg/kg xylazine (I.V) followed by 3 mg/kg ketamine (I.V) through the ear vein for rabbits and 1 mg/kg of xylazine (I.M) then followed by 5 mg/kg of ketamine (I.M) for cats (Hall et al., 2001).
The animals in this study were handled according to the guidelines of the Institutional Animal Care and the Research Ethics Committee of the Zagazig University.
Computed Tomography (CT) Scanning
Three-dimensional CT scanning was performed on both species after anesthesia at AL-Bayan Center of radiology and CT in Belbes, Sharkia Governorate, Egypt. CT images were taken without contrast medium using multi-slices CT system, which was capable of acquiring up to 32 slices per sec, with fast whole-body scan time of 0.5 sec, 50 kW X-Ray Generator, Multiple kV and mA techniques and 5.0 MHU X-Ray Tube. TOSHIBA 600HQ (third generation) Japan (Bohler et al., 2008).
Bone Preparations
Anatomical dissection by careful separation and cleaning of thoracic and lumbar vertebrae from all attached tissues. The vertebrae were prepared following the method of (Onwuama et al., 2012). The measurements were taken with a caliper and flexible meter to demonstrate the main differences between the two-animal species. The obtained vertebrae were photographed using a Sony Digital Camera, Dsc W810 20.1 MP.
Statistical Analysis
The data of the different anatomical parameters between the three groups were analyzed according to the SAS statistical system Package V9.2 (SAS, 2009) and these measurements were tested by using an Analysis of Variance (ANOVA) method. Data were presented as mean ± SEM, and the differences were considered significant at P ≤ 0.05.
The used anatomical nomenclature was based on Nomina Anatomica Veterinaria (NAV, 2017) whenever possible.
Results
Due to the paucity of specific osteological data, the present study focuses on the bony features of the thoracic and lumbar vertebrae and their articular surfaces in cat and rabbit to serve as a guide for further osteological, radiological, arthrological and surgical researches.
Thoracic Vertebrae
All comparative points in the current study had longer measurements in cat rather rabbit regarding the lengths of the back and loin except lumbar transverse process and lumbosacral space that were of notably increase length in rabbit (Table 1) (Fig 1A and 1B). These statistically different measurements reflected more thoracic and abdominal
Table 1: Anatomical measurements of the thoracic and lumbar vertebrae between rabbits and cats
| Anatomical measurements(cm) | Cats | Rabbits (1) | Rabbits (2) | P-Values |
| Length of thoracic region (back) | 15.0000±.12910 | 13.1000±0.12910 | 14.2500±0.10408 | 0.000 |
| Length lumbar region (loin) | 13.9000±.18708 | 12.0000±0.17795 | 13.2000±0.10801 | 0.000 |
| Length of back and loin | 28.6750±.12500 |
25.0250±0.11087 |
27.4750±0.13150 | 0.000 |
| Longest thoracic body |
1.3750±.04787 (13th v) |
1.7250±0.08539 (12th v) |
1.8250±0.08539 (13th v) |
0.005 |
| Shortest thoracic body |
0.9750±.08539 (5th v) |
0.5750±0.08539 (1st v) |
0.7375±0.04732 (1st v) |
0.013 |
|
Longest lumbar body (6th v) |
2.4000±.04082 | 1.7125±0.04270 | 1.8500±0.06455 | 0.000 |
|
Shortest lumbar body (7th v) |
1.7250±.03227 | 1.2125±0.03146 | 1.3500±0.06455 | 0.000 |
| Longest thoracic spine |
3.1125±.04270 (2nd v) |
2.7500±0.06455 (4th v) |
3.2500±0.06455 (4th v) |
0.001 |
|
Shortest thoracic spine (11th v) |
0.9625±.05543 | 0.6750±0.03227 | 0.8125±0.04270 | 0.005 |
|
Longest lumbar transverse process (6th v) |
2.7250±.11087 |
3.1000±0.10801 |
3.3250±0.08539 |
0.008 |
|
Shortest lumbar transverse process (1st v) |
0.9125±.04270 |
1.4000±0.04082 |
1.6000±0.08165 |
0.000 |
|
Widest diameter of thoracic vertebral foramen (1st v) |
0.9250±.03227 |
0.7100±0.02273 |
0.7850±0.00645 |
0.000 |
|
Narrowest diameter of thoracic vertebral foramen (13th v) |
0.7450±.01555 |
0.5650±0.00645 |
0.6500±0.00408 |
0.000 |
|
Widest diameter of lumbar vertebral foramen (6th v) |
0.9525±.00479 |
0.8050±0.00866 |
0.9050±0.00645 |
0.000 |
|
Narrowest diameter of lumbar vertebral foramen (1st v) |
0.7200±.00913 |
0.5075±0.00854 |
0.5150±0.10508 |
0.060 |
| Lumbosacral space |
0.04370± 1.3000 |
1.4000±0.04280 | 1.6000±0.0953 |
0.000 |
Rabbits (1): has 12 thoracic vertebrae value = mean±std. Error
Rabbits (2): has 13 thoracic vertebrae
index capacity in cat than rabbit which needed for more velocity.
The back region of cat had 13 thoracic vertebrae but varied in rabbit from 12-13 in numbers that contributed the dorsal boundary of the thoracic cage and articulated ventrolateraly with the ribs (Figs. 1C, D, E, F and 2B). The bodies of first two and last five cat thoracic vertebrae were compressed dorsoventrally, wider and longer than the mid-group (third to eight) (Fig. 2A and 2C). The first one and the last two or three thoracic vertebral bodies in rabbit were compressed dorsoventrally but longer and wider in the last three or four (Fig. 2D and 2F).
The diameter of the cat vertebral canal was gradually decreased caudally along the thoracic series; the vertebral foramen started wide triangular in the first vertebra and ended narrow in the last group (Figs. 2C, E and 3A, C). The intervertebral foramen was appeared wider in the cranial group than the caudal one (Figs. 1E and 3E). Both rabbit vertebral canal and intervertebral foramina were roomy in the cranial and caudal groups than the mid one (Figs. 1F and 3B).
The bodies of cat and rabbit thoracic vertebrae had convex cranial articular surfaces which in cat were centrally depressed especially at the terminal vertebrae, while the caudal articular surfaces were slightly concave (nearly flat) in cat and concave in rabbit (Figs. 3D, F and 4A). The cranial costal facets of thoracic vertebrae present on both sides of the cranial articular surface, except the last four in cat were more extended caudally, for articulation with the heads of the corresponding ribs (Figs. 2C and 4C). These facets were situated ventrally to the first two bodies, but cranially from third to tenth and directed more caudally in the last two or three bodies, in rabbit (Figs. 3D and 4B, D). The last four vertebrae in cat and last three or four in rabbit had no caudal costal facets on both sides of the caudal articular surface (Figs. 4C, D, F and 5A, B).
Both cranial articular processes in cat and rabbit were directed craniodorsally but seem to be extended more cranially preceding the level of cranial articular surface in rabbit preventing any interarcuate space. It represented by two
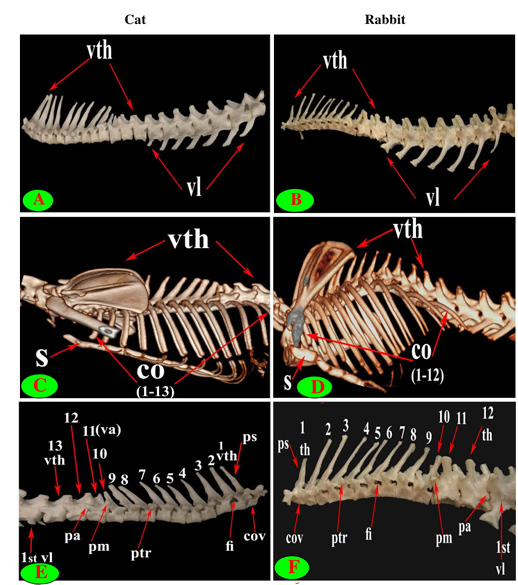
Figure 1: Vertebrae thoracicae (vth), Vertebrae lumbales (vl), Sternum (S), Costae (co), Vertebra anticlinalis (va), Processus spinosus (ps), Processus accessories (pa), Processus mamillaris (pm), Processus transversus (ptr), Foramen intervertebrale (fi) and Corpus vertebrae (cov).
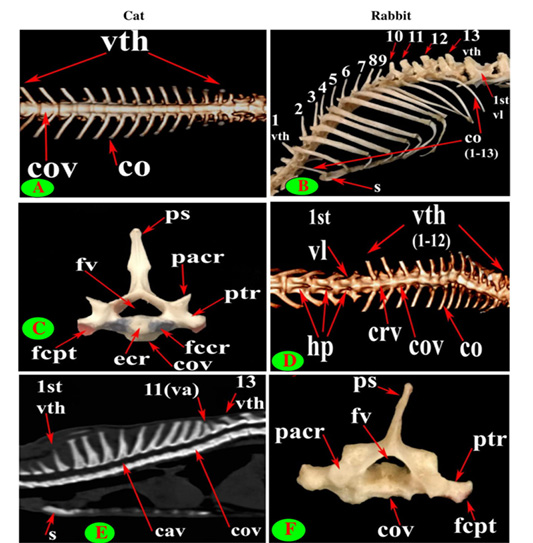
Figure 2: Vertebrae thoracicae (vth), Vertebrae lumbales (vl), Sternum (S), Costae (co), Vertebra anticlinalis (va), Processus spinosus (ps), Processus transversus (ptr), Corpus vertebrae (cov), Foramen vertebrale (fv), Fovea costalis processus transversi (fcpt), Extremitas cranialis (ecr), Fovea costalis cranialis (fccr), Processus articularis cranialis (pacr), Hypapophysis (infraspinous processes) (hp), Crista ventralis (crv) and Canalis vertebralis (cav).
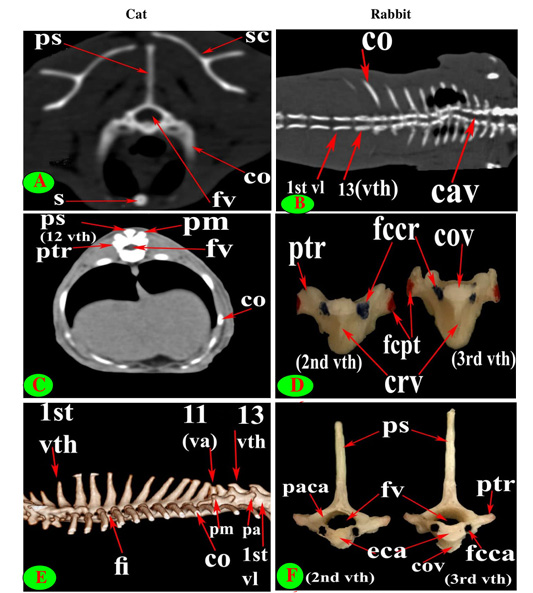
Figure 3: Vertebrae thoracicae (vth), Vertebrae lumbales (vl), Sternum (S), Costae (co), Vertebra anticlinalis (va), Processus spinosus (ps), Processus accessories (pa), Processus mamillaris (pm), Processus transversus (ptr), Foramen intervertebrale (fi), Corpus vertebrae (cov), Foramen vertebrale (fv), Fovea costalis processus transversi (fcpt), Fovea costalis cranialis (fccr), Crista ventralis (crv), Canalis vertebralis (cav), Scapula (sc), Processus articularis caudalis (paca), Extremitas caudalis (eca) and Fovea costalis caudalis (fcca).
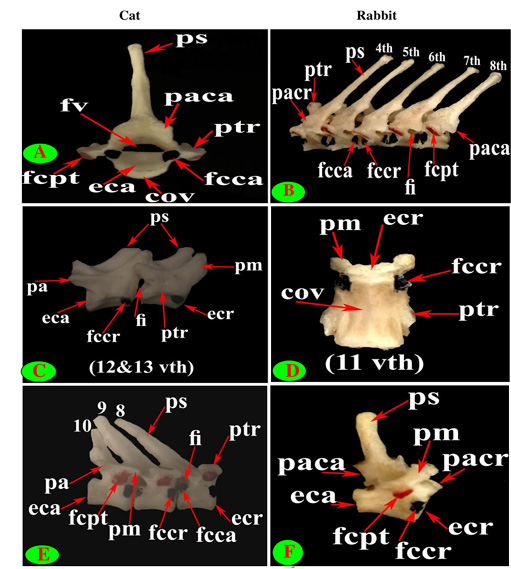
Figure 4: Vertebrae thoracicae (vth), Processus spinosus (ps), Processus accessories (pa), Processus mamillaris (pm), Processus transversus (ptr), Foramen intervertebrale (fi), Corpus vertebrae (cov), Foramen vertebrale (fv), Fovea costalis processus transversi (fcpt), Extremitas cranialis (ecr), Fovea costalis cranialis (fccr), Processus articularis cranialis (pacr), Processus articularis caudalis (paca), Extremitas caudalis (eca) and Fovea costalis caudalis (fcca).
close oval facets on the neural arch except the first two were larger and widely separated in cat (Figs. 2C and 5C), while in rabbit, it is was formed from two widely separated circular facets except the last two or three vertebrae were oval close facets on the neural arch (Fig. 5B and 5D). The caudal articular processes in the form of two close caudoventral facets situated at the root of spinous process and separated from it in the last three vertebrae in cat and last three or four in rabbit (Figs. 3F, 4A and 5A, B).
The spinous process of cat thoracic vertebrae was directed caudally till the anticlinal (11th) vertebra that had vertical spinous process and then inclined cranially in the last two (Figs. 1C, E; 2E and 3E). The rabbit thoracic spines divided into two directions; the first ten directed caudally and then directed cranially in the last two or three (Figs. 1D, F; 2B and 5F). All the cat spines were nearly equal with ill-defined gradual decrease in length, caudally except
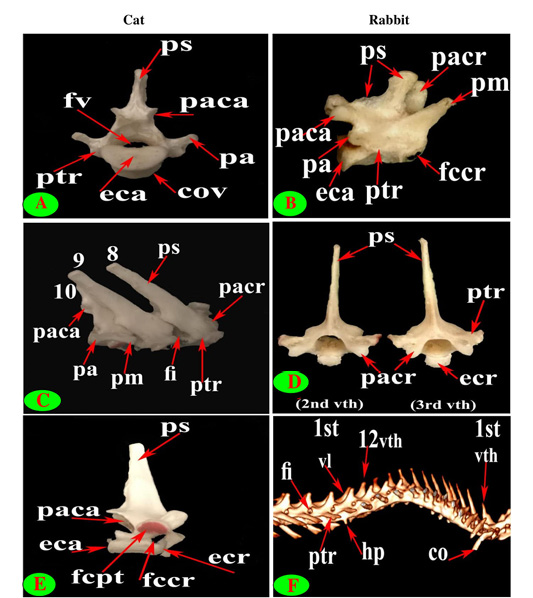
Figure 5: Vertebrae thoracicae (vth), Vertebrae lumbales (vl), Costae (co), Processus spinosus (ps), Processus accessories (pa), Processus mamillaris (pm), Processus transversus (ptr), Foramen intervertebrale (fi), Corpus vertebrae (cov), Foramen vertebrale (fv), Fovea costalis processus transversi (fcpt), Extremitas cranialis (ecr), Fovea costalis cranialis (fccr), Processus articularis cranialis (pacr), Hypapophysis (infraspinous processes) (hp), Processus articularis caudalis (paca) and Extremitas caudalis (eca).
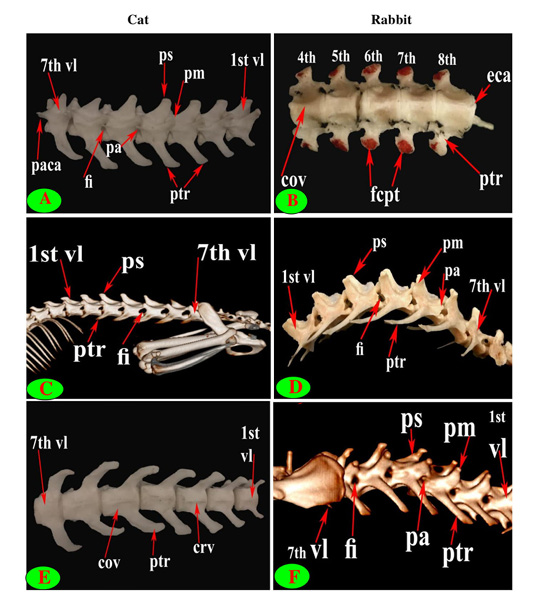
Figure 6: Vertebrae thoracicae (vth), Vertebrae lumbales (vl), Processus spinosus (ps), Processus accessories (pa), Processus mamillaris (pm), Processus transversus (ptr), Foramen intervertebrale (fi), Corpus vertebrae (cov), Fovea costalis processus transversi (fcpt), Crista ventralis (crv), Processus articularis caudalis (paca) and Extremitas caudalis (eca).
notable decline in-between the ninth and tenth (Figs. 1E, 2E, 3E and 4E). In rabbit, the spines gradually increase in length from the first to the third or fourth then gradually decrease caudally till the ninth and then continued with equal length to the last (Figs. 1F, 2B and 5F).
The supraspinous processes were long, narrow and ended by rounded summit in all cat vertebrae except the last three were shorter and wider. The spine had truncated summit in 12th and 13th but that of the eleventh (anticlinal) vertebra was in the form of triangular plate (Figs. 1E, 2E and 3E). The rabbit processes were thinner and ended by pointed summit in all vertebrae except the last four were short, broader and had truncated summit (Figs. 1F, 2B, 4B and 5B, F). All the cat and rabbit spines had cranial and caudal thin borders. The first four cat spines possessed a characteristic crest on both sides of their upper half ended distally by a tubercle (Fig. 2C).
The bodies of thoracic vertebrae devoid of infraspinous processes and represented by faint rough lines in first three and last two vertebrae in cat but this process in rabbit was well defined in the form of ventral crest in the first three and last four or five vertebrae (Fig. 2A and 2D).
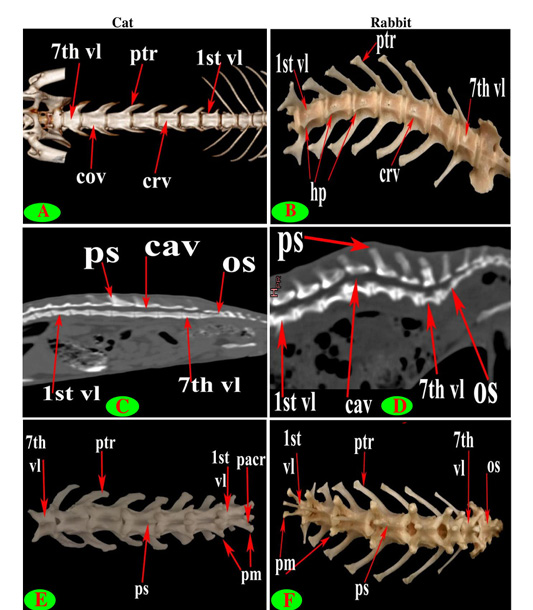
Figure 7: Vertebrae lumbales (vl), Processus spinosus (ps), Processus mamillaris (pm), Processus transversus (ptr), Corpus vertebrae (cov), Processus articularis cranialis (pacr), Hypapophysis (infraspinous processes) (hp), Crista ventralis (crv), Canalis vertebralis (cav) and Os sacrum (os).
The cat and rabbit transverse processes were paired, short and tuberous but stout in cat, it carried costal facets till the tenth one then replaced by low ridge without facets in the rest. The first four transverse costal facets were saddle-shaped then become rounded and convex in cat, while all rabbit tubercular facets were large saddle shape except the ninth and tenth that were small and convex (Figs. 1E, F; 4B, C, E, F; 5B, E and 6B).
The mamillary processes were small and paired that present in all thoracic vertebrae in cat and rabbit except the first two in cat (Figs. 1E, F; 2C; 4F and 5B). These mamillary processes in cat were located above the transverse process from third to eighth then bound with the accessory one in the form of bifid plate in the ninth and tenth vertebrae and separated from it to be beard on the cranial articular process in the last three (Figs. 1E, 4C, E and 5C). In rabbit, the mamillary processes were ill-defined above the transverse processes till be prominent and located in-between the transverse and the cranial articular processes in the tenth then beard on the cranial articular processes in the last three two or three thoracic vertebrae (Figs. 1F, 4F and 5B).
The cat accessory processes were separated caudally from the ninth and tenth mamillary processes to be in-between the caudal articular and transverse processes of last three vertebrae (Figs. 1E, 4C, E and 5C). Last two thoracic vertebrae in rabbit had accessory processes (Figs. 1F and 5B).
Lumbar Vertebrae
Seven lumbar vertebrae were arranged in cat and rabbit loin regions (Fig. 6A, C, E, D and F). The bodies were dorsoventrally flattened, especially the caudal group in rabbit, and also wider and longer than the thoracic one (Figs. 2D, 6E and 7A, B). The width and length of cat and rabbit lumbar bodies increased caudally except the last was shorter. Both cat and rabbit had greater lumbar vertebral canal than the thoracic vertebrae and increased in diameter caudally till the sixth vertebra (Figs. 3A, B and 7C, D).
The intervertebral lumbar foramina of the first group (1-3) in cat were narrower resembling the last thoracic group but these foramina be wider in the caudal one (4-6) (Figs. 1A and 6A, C). In rabbit, it seemed roomy along the series (Figs. 1B, F and 6D, F).
The cranial articular surfaces in cat and rabbit lumbar vertebrae were slightly convex while the caudal articular surfaces were slightly concave.
Both cat and rabbit lumbar cranial articular processes were directed craniodorsally represented by two close oval facets on the neural arch but the caudal articular processes in the form of two close caudoventral facets that were situated at the root of all spinous process except widely separated at the last (Fig. 7E and 7F).
The lumbar supraspinous processes were short. All cat spines were broad ventrally, narrower dorsally, cranially directed and ended by truncated summit resembling the last thoracic group except the last two in form of triangular spine (Fig. 6A and 6C). It appeared in rabbit in the form of cranial high part ended by truncated summit joined with caudal horizontal low transparent plate in all vertebrae except the last two without the horizontal part. All the spines have cranial and caudal thin borders (Fig. 6D and 6F).
The bodies of cat lumbar vertebrae had clear ventral crest which ill-distinct in the first and last vertebrae, while in rabbit had more prominent and pointed infraspinous processes (Hypapophysis) in first three lumbar bodies then continued caudally as a ventral crest (Figs. 2D, 5F, 6E and 7A, B).
The lumbar transverse processes were plate like and longer than that of the thoracic vertebrae and all had the same direction, cranioventrally. In cat and rabbit, it increased in length caudally except the last but the sixth was the longest. In cat, the first process was the shortest and narrowest while the last one was the broadest (Figs. 1A, 6A, C, E and 7A). In rabbit, the first process was the shortest but had the widest forked end while narrowest and thinnest at the last one (Figs. 1B, 6D, F and 7B, F).
The mamillary processes (Metapophysis) present in all cat and rabbit lumbar vertebrae beard on the cranial articular process (Figs. 6A, D, F and 7E, F).
The cat accessory processes (Anapophysis) were well-defined and crossed the caudal vertebral notch reaching the succeeding neural arch in all lumbar vertebrae except the last two (Figs. 1A and 6A). In rabbit, the latter processes were ill-defined and situated just ventral to the caudal articular process in all except the last two (Figs. 1B and 6D, F).
It is interesting to note that the number of thoracic (T) and lumbar (L) vertebrae was 13T/7L in 65% (13 rabbits) and 12T/7L in 35% (7 rabbits) of the total rabbits studied, while 100% of cats were 13T/7L.
The interlocking interarcuate articulation at the end of thoracic region (11-13) and lumbar region allow and facilitate the up and down movement (flexion and extension) but minimize the lateral movement that assist in forward speed of the cat. So difficult to insert a needle thus, the lumbosacral space was preferable in injection which was wide in diameter (distance between two ilia) and deep (Table 1).
Discussion
A basic understanding of the back and loin locomotor system in cat and rabbit especially the bones and their articulation features is extremely important for the animal movement, helping in species differentiation and adulteration that may occur in our country.
Currently, Computed Tomography (C.T.) plays an educational role to identify the thoracic and lumbar bony structures for using by veterinary radiologists, clinicians and surgeons (Hudson et al., 1994; Shojaei et al., 2003; Désirée et al., 2017).
Thoracic Vertebrae
In correlation with Miller (1964) in dog; Nickel et al. (1977) in carnivorous; Frank (2001) in marmosets; (Boyed et al., 1991; McMahon Karen, 2008) in cat and (Walter et al., 1988; Gore et al., 2001; Greenaway et al., 2001; Désirée et al., 2017) in rabbits, the current work recorded that, cat and rabbit had the same number of thoracic vertebrae (13) but sometimes 12 vertebrae in rabbit (Federico et al., 2013). Miller (1964) in dog added that, thoracolumbar region contained nearly constant 20 vertebrae; 13 thoracic and 7 lumbar with rare cases 21. The latter indicated that cat had longer back length in compare with rabbit of 12 thoracic vertebrae and even those of 13, as statistically mentioned inside the table, which give larger capacity of the thoracic cage to inspire more air during trotting.
Miller (1964) in dog, Dyce et al. (2009) in cat and Karen et al. (2007) in rabbit revealed that the bodies of thoracic vertebrae are increased in length caudally from the tenth, moreover the present investigation showed that the first two and last five bodies of cat thoracic vertebrae were wider and longer than the mid-group (3-8 vertebrae) while little difference in rabbit that constitute of the first one and the last three or four. Wagner (2004) mentioned that, the bodies of the thoracic vertebrae in common marmoset elongated towards the lumbar region while the spinous processes shortened and became broader.
In the same line, both cat and rabbit had wide vertebral canal and roomy intervertebral foramina in the fore group however the foramina continued wide caudally in rabbit but narrow in cat. Wide or narrow diameter of both canal and foramina reflected the degree of dorsoventrally compression of the vertebral bodies.
Concerning the convex cranial articular surfaces of thoracic vertebrae were centrally depressed in cat only but both species had concave caudal one.
Notably, the cranial costal facets were present in all rabbit thoracic vertebrae but absent in the last four vertebrae in cat. Cat and rabbit had no caudal articular costal facets in the last four vertebrae. That is dissimilar with that revealed with (Nickel et al., 1977) in carnivorous that revealed, the cranial costal facets became shallower caudally till the last thoracic one while a little difference with Miller (1964) in dog who reported that 11, 12 and 13 thoracic devoid of caudal costal one. Absence of caudal facets is very important to allow more free rotatory movement for the last group of asternal ribs.
Nickel et al. (1977) reported that the thoracic vertebral arches overlapped dorsally, therefore, no spatial interarcualia. In this regard, the obtained result and Miller (1964) in dog confirmed that cats resembling rabbits in its cranially directed articular processes which were close facets to each other except the first two processes in cat; but widely separated in rabbit except the last two or three. Rather than, the caudal articular processes were two close facets situated at the root of spinous process which separated from it in the last three vertebrae in cat and last three or four in rabbit. The closer facets of articular processes in cat permitted and support the forward rapid movement.
In contrary to cats, rabbits had no anticlinal vertebra (11th) however the same caudal direction of first ten thoracic spine and cranial orientation to the others, this corelated with that mentioned by Nickel et al. (1977) and Dyce et al. (2009) in cat and varied with Miller (1964) that recorded changing in length and direction after 7 or 8 vertebra. Although, a notable decline in length in-between ninth and tenth spines in both animals was observed in this result. Miller (1964) found the anticlinal vertebra in dog is T9 or T10.
A great correlation between cat and rabbit is the presence of long thoracic spine with rounded pointed summit in the fore group but shorter one with truncated summit in the caudal group.
It is interesting to note that, this study observed that collateral characteristic crests situated on the upper half of the first four spines in cat only.
Rabbit has a well-defined ventral crest in the cranial and caudal groups contrary to the cat. These results agreed with Nickel et al. (1977) in carnivorous.
The obtained result revealed that transverse processes carried a saddle shaped costal facets till the fourth vertebra in cat and till the eighth one in rabbit but replaced by convex rounded facets in the remaining vertebrae, that is also suggested to give more room of ribs during the abdominal respiration to get more inspired air to increase the speed.
In agreement with Dyce et al. (2009) in cat, our study revealed that the mammillary process in cat and rabbit was clearly obvious present on the cranial articular processes in the last three thoracic vertebrae, moreover, it is was located above the transverse process of the fore group. Nickel et al. (1977) added that, they fused with articular process forming Processus Mamilloarticulares. The latter is disagreed with Miller (1964) in dog who clarified that, the mammillary processes appeared at the 2 or 3 thoracic vertebrae as knob like eminence.
Additionally, the accessory processes were observed in both species in the last group (two or three vertebrae), thus is completely differed with that mentioned by Miller (1964) in dog that, the accessory processes appeared at the mid-thoracic region about the fifth or sixth.
Lumbar Vertebrae
The present work was similar to Miller (1964) in dog and Nickel et al. (1977) in carnivorous, that there were seven lumbar vertebrae that were possed longer and wider bodies and had wider vertebral canal than the thoracic region due to more compressed bodies. The length and width increased caudally except the last was the shorter. In the same line, Dyce et al. (2009) in cat added that, the bodies of lumbar vertebrae were twice length of those of the thoracic vertebrae, and also, Miller (1964) in dog clarified that the first thoracic body had the same length of the seventh.
The current work was completely agreed with Miller (1964) in dog and Karen et al. (2007) in rabbit, that clarified, although the number of lumbar vertebrae were only half that of the thoracic vertebrae, the lengths of the thoracic and lumbar regions were similar. Greenaway et al. (2001) in rabbit noticed that, the lumbar vertebrae were seven in 67.2% and six in 32.8% in the 64 rabbits studied, that wasn’t reported in our research.
Dislike the cat, all intervertebral foramina of lumbar vertebrae appeared roomy while only wide in the caudal group in cat thus to accommodate the lumbar swelling of spinal cord.
The same cranial convex and caudal concave articular surfaces were found in two species, which are conflict with that reported by Nickel et al. (1977) that the lumbar vertebrae had flat cranial and caudal extremities. Also, they had close oval craniodorsal or caudoventral facets representing the cranial and caudal articular processes except the last vertebra had widely separated caudal articular processes. The latter to adapt the widely separated two wings of the sacrum.
Concerning the cat movement, our work completely agreed with Miller (1964) in dog and Nickel et al. (1977) that said that, the lumbar articular processes had sagittal directed articular surface to restrict the lateral flexion of the lumbar region and allow the axial free movement (up and down movement).
Parallel to Miller (1964) in dog, the first five lumbar spines in cat ended by truncated summit and the last two had triangular one meanwhile the fore lumbar spine (1-5) in rabbit characterized by two divisions; cranial elevated one ended by truncated summit and caudal horizontal part. The latter part was absent in the last two lumbar vertebrae.
Interestingly, only rabbit had clear pointed hypapophysis (infraspinous process) in the first three lumbar bodies then represented by ventral crest in the caudal group as in cat.
Our study adapted with Miller (1964) in dog and (Dyce et al., 2009) in cat that mentioned that, the lumbar transverse processes were long, cranioventrally directed and overlapped on the proceeding vertebra. The present study added that, the first process was the shortest and sixth was the longest in both species but a notable difference in the seven process that it was the broadest in cat and thinnest in rabbit, thus might increase the abdominal space to improve the extension of the fully inspired lung. Miller (1964) and Nickel et al. (1977) is completely agreed with the latter but Nickel et al. (1977) added that the 5th and 6th vertebrae had the longest process.
The mammillary processes in cat and rabbit were well defined beard on the lumbar cranial articular processes, that closely similar to Miller (1964) in dog and Dyce et al. (2009) in cat. However, the accessory processes were present in both species in all lumbar vertebrae except the last two.
Contrast to Greenaway et al. (2001) in rabbit who added that, the number of thoracic (T) and lumbar (L) vertebrae was 12T/7L in 43.8%, 13T/6L in 32.8%, and 13T/7L in 23.4% in the 64 rabbits studied.
In the same line with Nickel et al. (1977) and (Dyce et al., 2009) in cat, the interarcuate space were very narrow in the thoracic and lumbar regions but it was large between the last lumbar and first sacral vertebra (lumbosacral space), so surgeons can inject their anesthesia through provide suitable site of injection or withdrawal of cerebrospinal fluid. Li et al. (2012) explained that, the rabbits divided into three groups for obtaining cerebrospinal fluid (CSF): skull drilling group, lumbar puncture group and atlanto-occipital membrane puncture group.
The present investigation illustrates clear significant differences present between cats and rabbits thoracic and lumbar vertebrae. Most of measurement parameters were significantly higher in cat rather than the transverse process and lumbosacral space in rabbit.
In conclusion, Cat has longer bony measurements unless transverse lumbar process and lumbosacral space were notably increase in rabbit. A good understanding of the thoracic and lumbar bony variations in cat and rabbit can facilitate surgical interventions and increase the veterinarian control for species adulteration in our country. Suitable site of injection of epidural anesthesia is the lumbosacral space which is wide in diameter and deep while so difficult to insert a needle into the interarcuate articulation. This articulation is interlocked at the end of thoracic and all lumbar vertebrae which help in maximize the up and down movement but minimize the lateral movement, that assist in forward speed of the cat needed to catch on the prey.
Acknowledgements
This work was supported in part by funding from the Egyptian Society for the Promotion of Science. The authors extend their appreciation and grateful to all staff members of Anatomy and Embryology Department, Faculty of Veterinary Medicine, Zagazig University, Egypt for their kind support during this study.
Conflicts of interest
The authors have no conflict of interest to disclose with respect to this work.
authors contribution
Both authors share in preparing the bony samples and writing the abstract and results. Dr. Sherif Khayri Abdelmoati Mohamed sent the animals for CT imaging, discussion and references writing, designing the final format of result text and photographs, statistically analyzing the data, revising all the manuscript, submission and approving the final version. Dr. Enas El-Hady wrote the introduction, material and methods and revised the manuscript.
References






