Advances in Animal and Veterinary Sciences
Research Article
Detection of Classical Swine Fever Virus Antigen and Nucleic Acid on Blood of Experimentally Infected Piglets
Sachin Dattatraya Raut1*, Kaushal Kishore Rajak1, Ravi Kumar1, Amol Ramdas Gurav2, Manu Mohan1, Dhanesh Valiyavalappil1, Dhanavelu Muthuchelvan1, Pranob Dhar3, Awadh Bihari Pandey1
1Classical Swine Fever Virus Laboratory, Division of Virology, Indian Veterinary Research Institute; 2Division of TAH, IVRI, Campus Mukteswar, Nainital 263 138, Uttarakhand, India; 3Division of Biological Standardization, IVRI, Campus Izatnagar-243122, UttarPradesh, India.
Abstract | Classical swine fever (CSF) is an economically important contagious viral disease, responsible for high mortality in young swine population. Classical swine fever virus (CSFV), the causative agent of CSF, belongs to genus Pestivirus under family Flaviviridae. Early detection of virus /genome is important for employing the disease containment measures. In present study, kinetics of antigen in experimentally infected piglets was determined by antigen ELISA and RT-PCR. Out of 12 sero-negative piglets, six were infected with virulent CSFV by intramuscular route, while other six were kept as control group. Piglets were regularly observed on the days post infection (dpi) for development of clinical manifestations of infection. Blood samples were collected on 0, 3, 6, 9, 12, 15, 18 and 21 dpi or till animal succumb of disease. Samples were screened by commercially available antigen ELISA and RT-PCR, for detection of viral proteins and nucleic acid to confirm the disease, prior to onset of clinical signs. Typical symptoms of disease were observed in the infected piglets on 10-12 dpi. Presence of CSFV antigen in blood sample from day’s 6th to7th post infection and viral nucleic acid on 6th and 9th dpi were confirmed by antigen ELISA and RT-PCR respectively. It was concluded by the present study that CSFV antigen can be detected even before the appearance of clinical signs. Such early detection can be used for field samples to test their repeatability and to prove as tools to control the spread of disease and to minimize economic losses.
Keywords | CSFV, Experimental Infection, RT-PCR, ELISA
Editor | MA Ramakrishnan, M.V.Sc., PhD., Post Doc (USA), Senior Scientist, Division of Virology, Indian Veterinary Research Institute, Mukteswar, Nainital, Uttarakhand, India.
Special Issue | 5(2015) “Emerging, Re-Emerging and Important Infectious Diseases of Animals”
Received | January 24, 2015; Revised | February 18, 2015; Accepted | February 20, 2015; Published | March 03, 2015
*Correspondence | Sachin Dattatraya Raut, Indian Veterinary Research Institute, Nainital, Uttarakhand, India; Email: [email protected]
Citation | Raut SD, Rajak KK, Kumar R, Gurav AR, Manu M, Dhanesh VV, Muthuchelvan D, Dhar P, Pandey AB (2015). Detection of classical swine fever virus antigen and nucleic acid on blood of experimentally infected piglets. Vet. Sci. 3(5s): 1-6.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.5s.1.6
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Raut et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
CSF is a highly contagious viral hemorrhagic disease of domestic pigs causing serious economic losses (Paton and Greiser-Wilke, 2003). The causative agent of disease, CSFV, belongs to the Pestivirus genus of the Flaviviridae family (Wengler, 1991). In India, most of the outbreaks have been reported from North-East states, where a large number of country’s pig population is reared. The mean prevalence of CSFV antibodies in different parts of India has been found to be 63.3%. On the other hand 76.7% of the suspected samples were found to contain CSFV antigen (Nandi et al., 2011). Virus is transmitted oronasally by direct or indirect contact with infected pigs or by feeding virus contaminated feed (Fritzemeier et al., 2000; Moennig et al., 2003). CSF diagnosis in a herd in the early phase of infection has got paramount importance for both economical and epidemiological points of view (Dewulf et al., 2004; Lipowski et al., 1996). The standard procedure for the detection of CSFV infection is based on virus isolation, which is then confirmed by the use of antigen detection ELISA and RT-PCR (McGoldrick et al., 1999; De Smit, 2000). RT-PCR has been found to be the most sensitive method for detection of CSFV (Le Dimna et al., 2008). During the phase of CSF epidemics, when a large number of samples need to be screened, antigen detection ELISA was found promising (Shannon et al., 1993). A wide variety of samples are suitable for disease diagnosis, which helps in detection of viral antigen in early phase of infection which reduces the time of diagnosis and spread of disease. The aim of present study was to detect CSF virus from blood samples of experimentally infected piglets by antigen ELISA and RT-PCR. This will help to confirm the infection of CSFV well before onset of the clinical signs and symptoms and to implement control strategy more effectively.
Materials and Methods
Experimental Animals
Twelve apparently healthy seronegative for CSFV white large Yorkshire (10-12th week’s age and 20-30 kg in weight) piglets of either sex were used in present study. Seronegativity was tested using commercially available ELISA kit (IDEXX CSFV antibody test kit). All the animals were housed comfortably and provided feed and water ad libidum.
Virulent Virus
Virulent CSFV (Mhow/2005) being maintained by needle passage in pigs, at Division of Biological Standardization, IVRI, Izatnagar was used for experimental infection. Identity of the virus was re-confirmed by RT-PCR before inoculating to the animals.
Experimental Infection and Sample Collection
Twelve ear tagged seronegative piglets were divided into two groups (A and B), of six piglets each. Both groups were housed and handled separately during entire experimental period. Six piglets from group A were inoculated intramuscularly following OIE guidelines, with an amount of virus corresponding to 105 PID50 (median pig infectious dose), with 5ml of 10% spleenic suspension of virulent CSF virus. Onset of disease and clinical symptoms were observed daily post infection. Piglets of group B were kept as healthy control. All the animals were observed to evaluate clinical score based on body temperature (Peak level: +++) and typical symptoms of CSF (++: huddling, inco-ordination, +++: reddening skin at the base of ear, inner side of abdomen, legs etc.) till survival/ completion of experiment. Blood samples were collected from all the animals in vacutainers containing EDTA on 0,3,6,9,12,15,18 and 21 dpi. All the samples were placed into a freezer (-80°C) until time for examination.
Detection of CSFV Antigen and Nucleic Acid
CSFV antigen from blood samples was tested by commercially available priocheck CSFV Ag ELISA following manufacture’s protocol; whereas viral nucleic acid was detected by RT-PCR. RNA Extraction was done by using commercial kit (Macherey-Nagel Nucleospin RNA Blood Midi) and quantity and purity of total RNA was checked by nanovue spectrophotometer. Approximately 1000ng of RNA was reverse transcribed using RevertAidTM First Strand cDNA Synthesis Kit (M/s MBI Fermentas Life Sciences, Maryland, USA). Primers for amplification of CSFV 5’UTR gene were used as per published reference (Paton et al., 2000). The stepwise cyclic conditions were: step I, 1 cycle, 95°C for 3 min; step II, 35 cycles, 95°C for 30 sec, 50oC for 45 sec., 72oC for 1 min; step III, 72oC for 10 min. The PCR products were analysed by electrophoresis using 1.5 % agarose gel along with molecular weight marker.
Results
Screening of Piglets for Seronegativity
Maximal colour development at the end of reaction in blocking ELISA using pre-infected sera confirmed the absence of CSFV specific antibodies. These observations were interpreted by calculating the blocking percentage value, which was found to be less than 30 percent (<30%). This indicated that piglets were seronegative against CSFV.
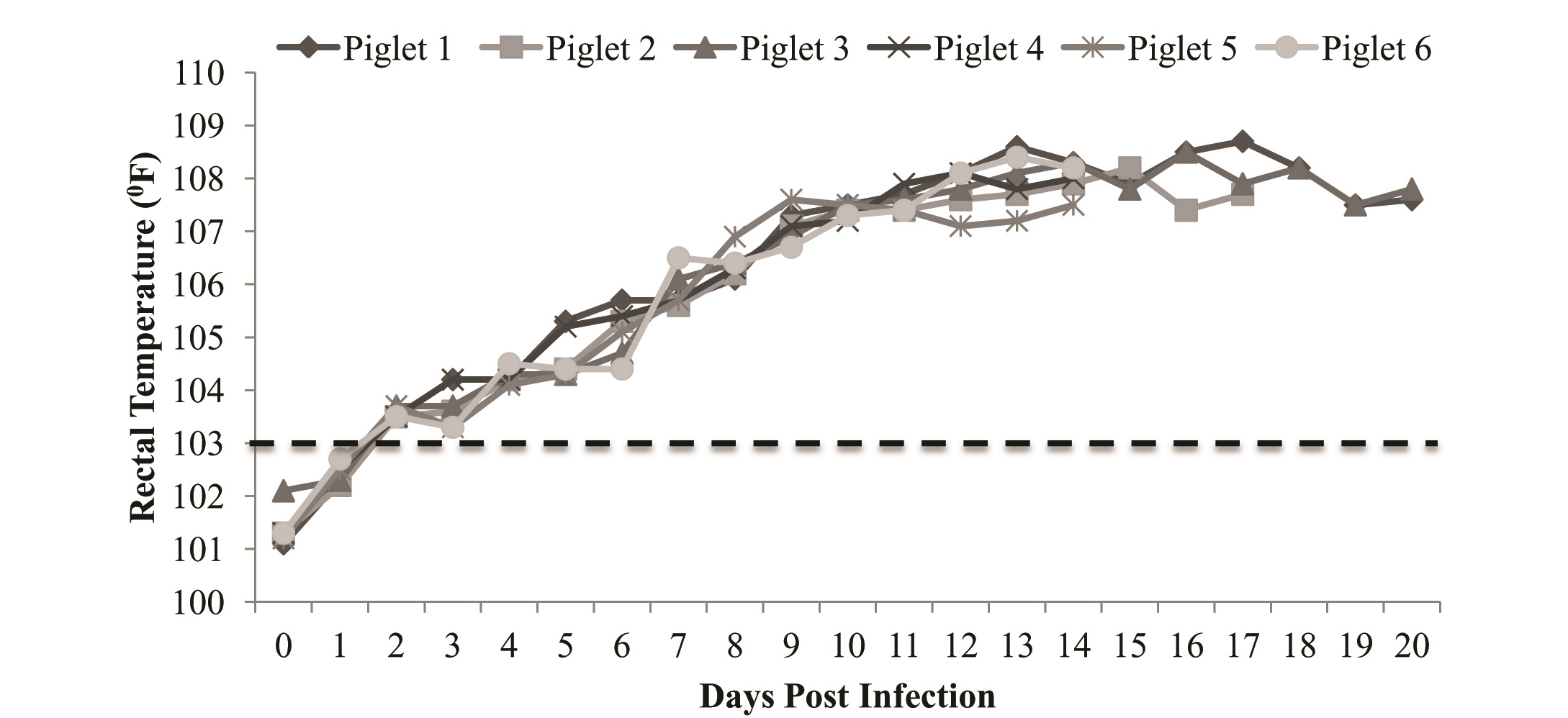
Figure 1: Rectal temperature of experimentally infected piglets showing rise in temperature from 3-4th dpi with peak on 14-15th dpi
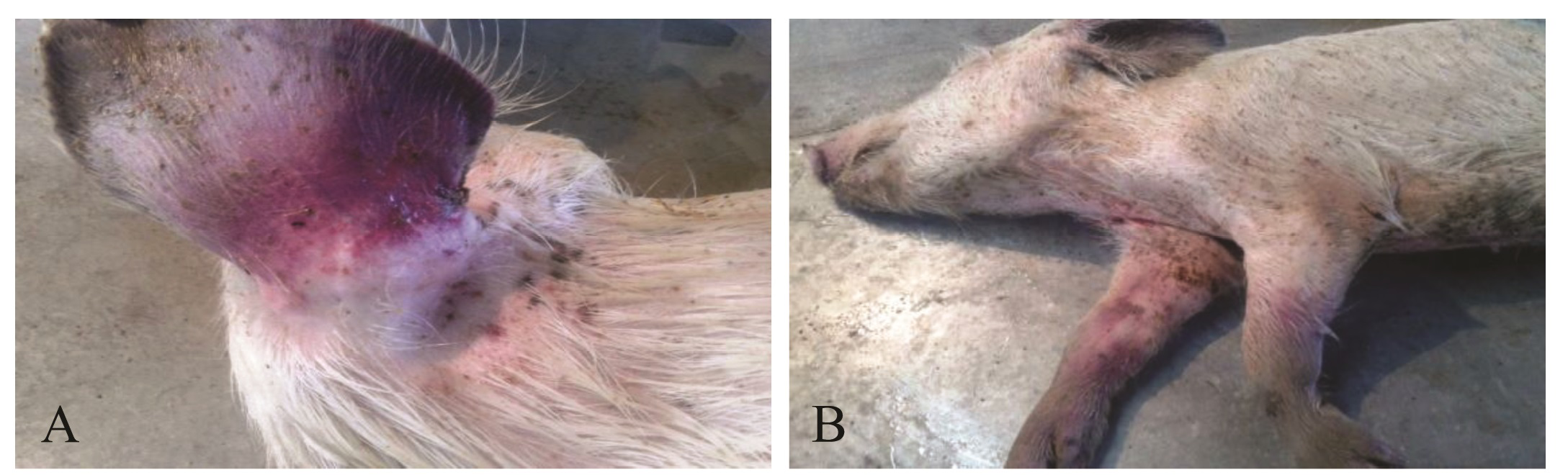
Figure 2: Clinical signs in experimentally infected piglets: A. Cyanosis of ear base; B. Dead carcass with cyanotic discoloration at inner side of abdomen and legs
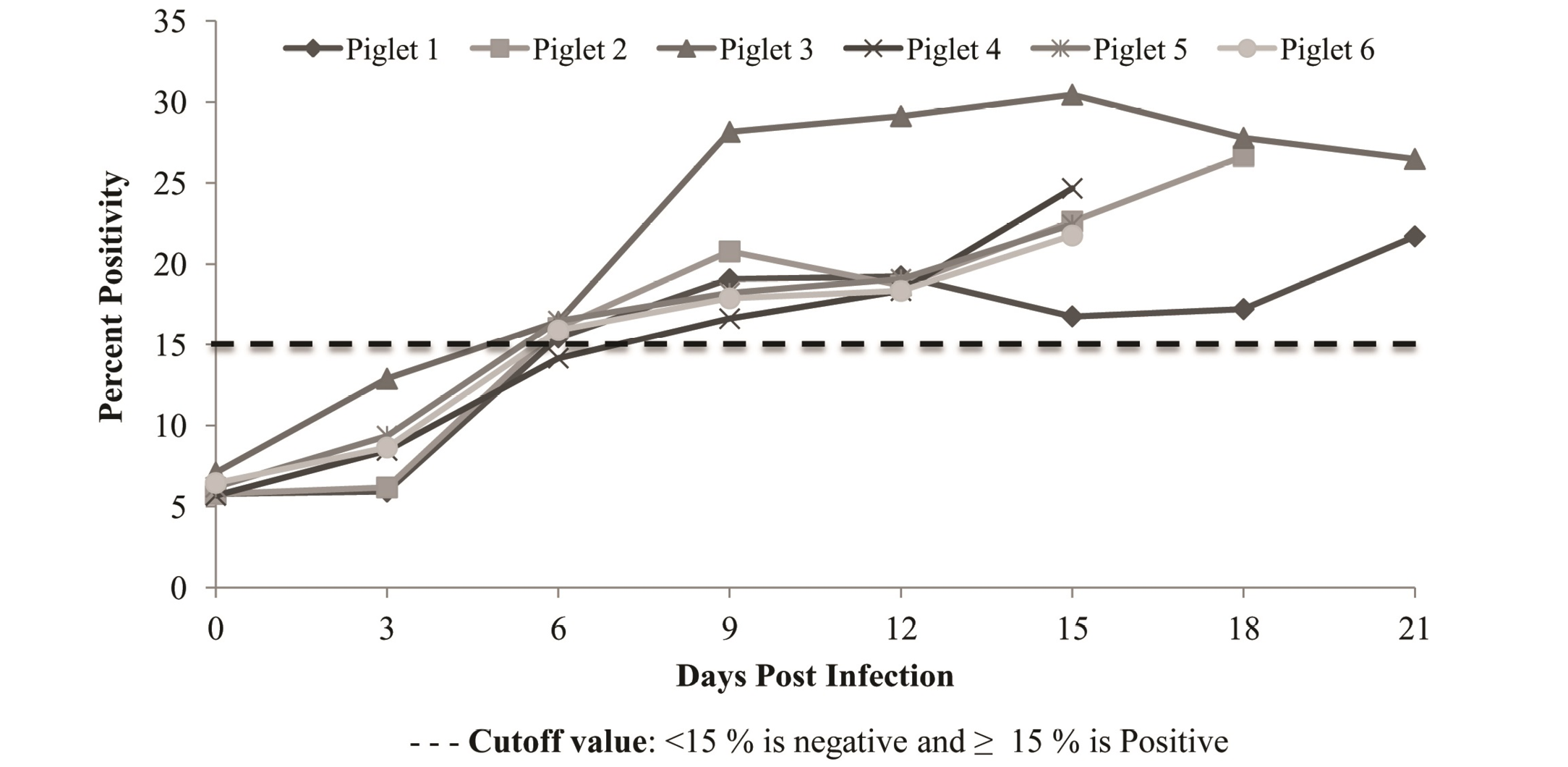
Figure 3: Detection of CSFV antigen in blood of experimentally infected piglets using priocheck CSFV Ag. Kit. All the animals were found to be positive from 6th dpi with gradual increase in titre till death
Experimental Infection
All the piglets of infected group showed increase in rectal temperature; which reached to peak level (106oF-108oF) after 5-6 dpi of virus (Figure 1). Typical symptoms of CSF (huddling, inco-ordination, reddening skin at the base of ear, inner side of abdomen, legs etc.) were observed10-12 dpi onwards (Figure 2) and all the piglets died in between 14-21 dpi. During postmortem, typical lesions (hemorrhages in the visceral organs like trachea, bladder, intestinal mucosa and caecum) of CSF was observed. Piglets of control group were found normal till the end of the experiment.
Detection of CSFV Antigen and Viral RNA in Blood
CSFV antigen was initially detected on day’s 6th-7th post infection and gradually increased till last day/ the day of death (Figure 3). Blood samples from control group were found to be negative for CSFV antigen.
Majority of infected piglets exhibited viral nucleic acid in the blood on 9th dpi by amplification of 5’UTR; whereas on 6th dpi, amplification noticed in blood sample of some infected piglet (Table 1).
Table 1: Evaluation of CSF disease vis-à-vis antigen kinetics in blood of experimentally infected piglets
|
Piglet no. |
Parameters |
Days post infection |
|||||||
|
0 |
3 |
6 |
9 |
12 |
15 |
18 |
21 |
||
|
1 |
Clinical Score |
- |
- |
++ |
++ |
++ |
+++ |
+++ |
+++/* |
|
ELISA |
N |
N |
P |
P |
P |
P |
P |
P |
|
|
RT-PCR |
N |
N |
N |
P |
N |
N |
N |
N |
|
|
2 |
Clinical Score |
- |
- |
++ |
++ |
++ |
+++ |
+++/* |
|
|
ELISA |
N |
N |
P |
P |
P |
P |
P |
||
|
RT-PCR |
N |
N |
P |
P |
N |
N |
N |
||
|
3 |
Clinical Score |
- |
- |
++ |
++ |
++ |
+++ |
+++ |
+++/* |
|
ELISA |
N |
N |
P |
P |
P |
P |
P |
P |
|
|
RT-PCR |
N |
N |
N |
P |
N |
N |
N |
N |
|
|
4 |
Clinical Score |
- |
- |
++ |
++ |
+++ |
+++/* |
||
|
ELISA |
N |
N |
P |
P |
P |
P |
|||
|
RT-PCR |
N |
N |
P |
P |
N |
N |
|||
|
5 |
Clinical Score |
- |
- |
++ |
++ |
+++ |
+++/* |
||
|
ELISA |
N |
N |
P |
P |
P |
P |
|||
|
RT-PCR |
N |
N |
P |
P |
N |
N |
|||
|
6 |
Clinical Score |
- |
- |
++ |
++ |
++ |
+++/* |
||
|
ELISA |
N |
N |
P |
P |
P |
P |
|||
|
RT-PCR |
N |
N |
P |
P |
N |
N |
|||
- : No disease +: Mild; ++: Moderate; +++: Severe; *: Death; N: Negative; P: Positive
Discussion
The infected pigs shed large amount of virus in saliva and smaller quantities in urine, nasal and lachrymal fluids that leads to CSF epidemics (Edwards, 2000). Thus, early tracing of possibly infected herds is focused on the detection of CSFV antigen (Shannon et al., 1993) rather than CSFV antibodies and further confirmed by RT-PCR technique, which is specific diagnostic assay that can complement classical serological procedures (Depner et al., 2007). Virus isolation is standard procedure for CSFV detection (Terpstra, 1996), however not suitable during outbreak conditions since, it is labor intensive and time consuming for results (Terpstra and de Smit, 2000; Dewulf et al., 2004). Early detection of CSFV during outbreak condition helps field veterinarian to eliminate infection from the pig population. Present study initiated with illustration of absence of CSFV specific antibodies in 10-12th week’s age old piglets and infected with virulent CSF virus. Pyrexia is one of the first signs of CSF and is accompanied by viremia. During experimental and horizontal infection of CSF virus, there is variable latency period from inoculation to viraemia and it was reported that, first viraemia is detected at 4 dpi in experimental infection whereas on 12 & 14 dpi in horizontal infection (Laevens et. al., 1998). In this study, infected piglets, showed initiation of increase in rectal temperature on 3-4 dpi followed by peak level (1060F-1080F) on 14-15 dpi. Piglets remain vireamic during 6-9 dpi. Similar findings have been reported that pigs get infection by oro-nasal route, develop high fever on 4-6 dpi (Dahle et al., 1991, Dewulf et al., 2004) and viremia was detected between the first and third day from the beginning of the febrile conditions in infected animals (Milanov et al., 2002). In infected piglets, course of the disease was influenced by the virulence of the virus and symptoms may vary hence, the antigen ELISA, based on the detection of viral antigen (Depner et al., 1995) and RT-PCR become helpful for rapid detection of CSFV (Le Dimna et al., 2008). In infected piglets, course of the infection was evaluated by using antigen ELISA and confirmed by RT-PCR technique. CSFV antigen was found in 33 blood samples of infected piglets, collected on 6-7th dpi onwards. It was found that 5’UTR region of CSFV was amplified in 10 samples, collected from infected animals on day 6th and 9th post infection only. This is in agreement with Jasna et al. (2007) who reported that CSFV antigen (viraemia) is detected on 7th and 9th dpi by ELISA in piglets of different age group (28, 35, 44 and 54 days old) exposed to contact infection of CSFV and these findings were confirmed by applying RT-PCR technique, the presence of viral RNA was detected only in 3 piglets from blood samples. Hence detectable level of CSFV antigen was observed on day 6-7th post infection and gradually increased till death. The typical symptoms of CSF (Anorexia, conjunctivitis, in-coordination, diarrhea, reddening of the base of ear etc) observed 10-12 dpi onwards and was found to be similar to those reported by Cariolet et al. (2008). All the piglets showed almost same pathogenesis and no individual variation could be noticed. This may be due to similar origin of piglets. Postmortem examination revealed typical lesions (hemorrhages in the visceral organs like kidney, trachea, bladder, intestinal mucosa and caecum) of CSF. Hence it can be concluded that choice of laboratory test depends on the goal, in this study the antigen ELISA and RT-PCR technique found to be promising to detect early infection of CSF virus in experimentally infected piglets well before clinical manifestations. Hence the present study will be helpful to reduce the time of diagnosis and spread of disease which ultimately leads to effective control of the disease.
Acknowlegement
The authors are grateful to Director of the Institute for providing the necessary facilities to carry out this work. Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi is highly acknowledged for providing necessary funds under DBT-CSF network project to carry out this study.
Conflict of interest
The authors declare that they have no conflict of Interest.
References





