Advances in Animal and Veterinary Sciences
Short Communication
Evaluation of Silver Nanoparticle Mediated Reduction of Neovascularisation (Angiogenesis) in Chicken Model
Rekha Khandia1*, Ashok Munjal1, Raj Sonam Bangrey1, Reena Mehra1, Kuldeep Dhama2, Naresh Chandra Sharma1
1Department of Biochemistry and Genetics, Barkatullah University, Bhopal, Madhya Pradesh – 462026; 2Division of Pathology, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly, Uttar Prades-243122, India.
Abstract | Neovascularisation is a key event during the growth of solid tumor and the process is called angiogenesis. Tumor cells demands higher oxygen and their glucose requirement is also higher than normal cells, and during the growth of solid tumor new blood vessels also arise rapidly to fulfil the raised requirement of nutrients. Hence targeting the formation of new blood vessel formation leading to deprived nutrition and oxygen supply will lead to inhibited growth of tumor. In the present study, silver nanoparticles were manufactured by green synthesis using Azadirachta indica (neem) leaves and its effect on process of neovascularisation was evaluated. Bioconversion from silver nitrate solution to silver nanoparticles included addition of AgNO3 (1mM) to fresh leaf broth of Neem and the conversion was allowed to take place for 4 hrs. with continuous agitation at 80 rpm. Formation of silver (Ag) nanoparticle was visualised by change in colour from pale yellow to dark brown. Nanoparticles when subjected to developing embryonated eggs of chicken were found to reduce the number of viable blood vessels with reduction in number of vessel branch points in chorioallantoic membrane leading to the death of embryo. Silver nanoparticles prepared through bioconversion in the study, successfully reduced angiogenesis in embryonated chicken model and this anti-angiogenic property of Ag nanoparticles, can be explored as a potential therapeutic against pathological angiogenesis and solid tumors by targeting the vasculature.
Keywords | Anti-angiogenesis effect, Silver nanoparticles, Azadirachta indica, Green synthesis, Chorioallantoic membrane, Chicken model, Vasculature targeted therapy
Editor | Muhammad Zubair Shabbir, Assistant Professor, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 21, 2015; Revised | May 27, 2015; Accepted | May 27, 2015; Published | June 02, 2015
*Correspondence | Rekha Khandia, Barkatullah University, Bhopal, Madhya Pradesh, India; Email: [email protected]
Citation | Khandia R, Munjal A, Bangrey RS, Mehra R, Dhama K, Sharma NC (2015). Evaluation of silver nanoparticle mediated reduction of neovascularisation (angiogenesis) in chicken model. Adv. Anim. Vet. Sci. 3(7): 372-376.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.7.372.376
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Khandia et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Angiogenesis is a normal part of growth and healing. Angiogenesis, which is the formation of new blood vessels from pre-existing ones, is a complicated balance between stimulating and inhibiting factors. Though involved in growth, uncontrolled angiogenesis often results in pathological conditions like rheumatoid arthritis, diabetic retinopathy, solid tumor, hemangioma and psoriasis. To overcome pathological angiogenesis several therapeutics have been developed by employing monoclonal antibody (Krämer et al., 2007) like Bevacizumab and vitaxin, inhibitors of angiogenic inducers or their receptor (Jänne et al., 2007), inhibitors of endothelial cell intracellular signalling (Sonpavde et al., 2008) etc. Nano-biotechnology is presently one of the most dynamic disciplines of research in contemporary material science. Different nanoparticles have been shown to modulate angiogenesis. Copper nanoparticles have been shown to have proangiogenic effect (Mroczek-Sosnowska et al., 2015) where, gold (Arvizo et al., 2011), perflurocarbon nanoparticles (Das et al., 2013), carbon allotropes (Grodzik et al., 2011) etc. have been shown to inhibit angiogenesis. The unique structure of the crystal lattice of silver (Ag) allows it to store atomic oxygen inside the octahedral holes of Ag (0) and probably influences oxygen level in environment (Hamburger and Hamilton, 1951) and possibly this property attribute to the anti-angiogenesis. Nanoparticles of various materials, such as Pt (Platinum), Ag (Silver), Au (gold), PbS (lead sulphide) and fullerene, have been reported to be produced by evaporation/condensation technique (Magnusson et al., 1999; Ranjan et al., 2013). Now a days there is an increasing number of reports using green technology i.e. usage of plant materials to synthesize Ag nanoparticles (Forough et al., 2010; Okafor et al., 2013; Banerjee et al., 2014; Elumalai et al., 2014).
Synthesis of green silver nanoparticles using plant extracts is a very simple and cost-effective way that eliminates the possibility of environmental hazards, and method has been described by several researchers (Tripathi et al., 2009; Lalitha et al., 2013; Shukla et al., 2013; Banerjee et al., 2014). In present study, silver nanoparticles were prepared by green synthesis method using neem (Azadirachta indica) extract and the resulting nanoparticles were evaluated for their effect on the process of angiogenesis in chicken embryonic chorioallantoic membrane (CAM) model.
The fresh neem (Azadirachta indica) leaves were thoroughly washed, air dried and finely cut with distilled water. Twenty grams of the leaves were added to 100 ml of de-ionised water and boiled in a water bath to bring the volume of extract to 50 ml. The extract was filtered through 0.45 µM filter. Silver nanoparticles were prepared by method described by Tripathi et al. (2009) with minor modifications. The prepared neem leaf broth was mixed with AgNO3 in 1:4 ratio to bring the final concentration of AgNO3 to 0.25 mM, 0.5 mM, 1 mM and 1.5 mM, respectively. The pH of the solution was adjusted to 8, using 5% solution of NH4OH. The nanoparticles formation was allowed to take place for 16 hrs at ambient room temperature with agitation at 80 rpm. A total of 100 ml reaction mixture was prepared. The color changed from transparent to dark brown is evidence of reduction of silver nitrate solution to silver nano-particles. The suspension was centrifuged at 1200 rpm for 30 min to obtain nanoparticles. A loose greyish-brown pellet of nanoparticles appeared. Carefully the supernatant was removed with help of pipette and 5 ml of nanoparticle suspension was allowed to retain. Chicken embryonated eggs aging 9-11 days (n=20) were obtained from State Poultry Farm, Raisen Road, Bhopal, Madhya Pradesh, India. These were cleaned with 70% alcohol and divided into control and treatment groups comprising of 10 eggs each. Two hundred microliters of nanoparticles with antibiotic (ampicillin 50 µg/ml, Streptomycin 10 µg/ml), and antimycotic solution (amphotericin 10 µg/ml) was overlaid on the chorioallantoic membrane (CAM) of the chicken embryos of the treatment group through air sac. Control group was treated by same volume of antibiotic containing deionised water with no nanoparticle treatment. All the eggs of both the groups were incubated for 72 hrs. at 37°C in a humid chamber. After 72 hrs. the egg shells were opened and CAM were examined grossly as well under microscopic field vascular branch points, an indicator of amplitude of angiogenesis.
Synthesis of green silver nanoparticles using plant extracts is a very simple and cost-effective way. Usage of Ag nanoparticles may be preferred than gold nanoparticles in addressing angiogenesis due to availability of low cost salts. The particle size of Ag nanoparticles may be controlled by changing the reaction temperature, leaf broth concentration and AgNO3 concentration (Raut et al., 2009). In the present study, maximum nanoparticles formation occurred with 1 mM AgNO3, as evidenced by change in color from pale yellow to dark brown. The same concentration has been found optimum by Raut et al. (2009) and Gavhane (2012).
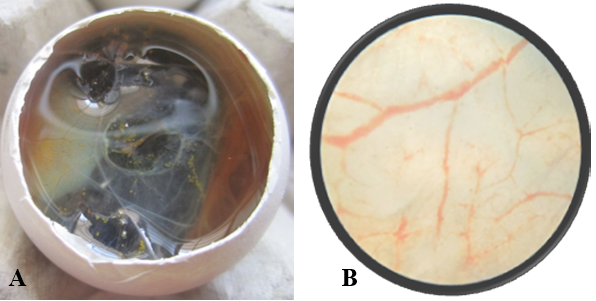
Figure 2: A) Nanoparticle treated Egg; B) Nanoparticle treated Egg- Microscopic view- 5X, No. of branch points=9
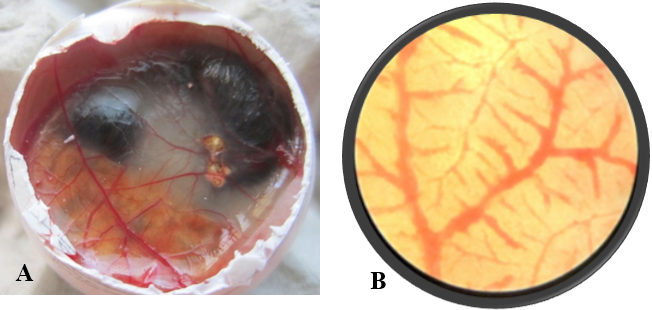
After opening the shell of the eggs of control and treated groups, in control group (Figure 1A), all the 10 embryos were live, very active and they remained alive for one hour, and showed movement upon touch, even after excising from the egg. A well arbourized, vascular system was observed. All the blood vessels were filled with blood hence, were red in coloration. In nanoparticles treated group (Figure 2A), 6 embryos were found dead and remaining 4 were showing very sluggish movement and even after piercing with sharp object, they did not respond well, indicating the condition before death. Also the blood vessels were thin with less degree of vascularisation (Figure 2A). The chorioallantoic membranes (CAM) of chicken eggs were examined under 5X microscopic field. Per egg, 10 fields were visualized and an average of number of vascular branch points was calculated. In control group, average 42 branch points were visible (Figure 1B), while in nanoparticle treated group only 9 branch points (Figure 2B) were visible. This difference is statistically different (p<0.001) as determined by Student t test (Pearson, 1939). The reduced number of branch points is indicative of angiogenesis reduction.
Silver nanoparticles have been known to reduce vasculature (Kang et al., 2011; Baharara et al., 2014). In the present study, death of embryos of chicken was observed in in-ovo model. As blood vessels are the source of nourishment and oxygen supply, hence are essential for life and during the process of growth and development these play a major role. Poor vasculature in Ag nanoparticles treated group might be responsible for death due to deprived nourishment and oxygen supply. Neovascularization is a process, which is rare in adults and appear only during wound healing or menstrual cycle in women or otherwise in pathological conditions (Miyazaki et al., 2015). Though in the present case Ag nanoparticles caused death of embryo, but it also reduced the vascularisation efficiently and significantly as evidenced by reduced number of branch points on CAM in treated eggs, an indicative of angiogenesis reduction. Gold nanoparticles are also known to reduce angiogenesis significantly by inhibiting the proliferation, migration and tube formation, possibly by blocking vascular endothelial growth factor (VEGF) mediated signalling pathway (Kalishwaralal et al., 2011). Similarly, silver nanoparticles also inhibit formation of new blood micro vessels through VEGF inhibition in the mouse Matrigel plug assay (Gurunathan et al., 2009). Also, silver nanoparticles are reported to alter vascular permeability induced by VEGF, interleukin (IL)-1β, in retinal endothelial cells (Sheikpranbabu et al., 2009). Disturbance in cytoskelatal organization was also observed followed by nanoparticles treatment. The effect of nanoparticle could be attributed to possible interaction of silver nanoparticles with proteins and thiol group containing enzymes (glutathione, thioredoxin, Superoxide dismutase (SOD) and thioredoxin peroxidise etc.), which are involved in nullifying the oxidative stress of ROS (Reactive Oxygen Species), generated by mitochondrial energy metabolism (Mohammadzadeh et al., 2012). Thus accumulation of ROS, initiates inflammation and destruction of mitochondria leading to release of cytochrome C and programmed cell death event to happen (Chen et al., 2008).
Although, there are various reports of nanoparticles mediated angiogenesis suppression, evidences of neem extract (ethanolic) mediated anti-angiogenesis is documented in Human Umbilical Vein Endothelial Cells (HUVECs) with evidence of fragmented nuclei and abnormally small mitochondria with dilated cristae. On the basis of genome-wide gene expression microarray analysis of HUVECs and quantitative real-time PCR indicate dselective upregulation of HMOX1, ATF3, and EGR1. HMOX1, an enzyme degrades heme to carbon monoxide, iron, and biliverdin has been documented to play crucial role in cellular defense against stressful conditions (Loboda et al., 2008). A large number of plant extracts and pharmacological compounds (e.g., green tea, curcumin including neem) have been demonstrated to induce HMOX1 (Aziz et al., 2010; Wu et al., 2011). ATF3 is a novel stress-activated regulator of p53 protein stability and function (Whitlock et al., 2011) and over-expression of ATF3 leads to p53 activity and induce apoptosis of cells (Tian et al., 2009). Neem extract increase EGR1, but exact role of it against angiogenesis is still unknown. Hence it could be possible that anti-angiogenic property of biogenic silver nanaoparticle is due to combinatorial effect of neem extract and silver nanoparticles both. Effectiveness of ethanolic extract of neem is well documented in anti-angiogenesis (Mahapatra et al., 2012) and mammary carcinogenesis inhibition (Arumugam et al., 2013). Aqueous extract of Neem leaf is also found to be effective in inducing apoptosis in leukemia and colon cancer cell in vitro (Roma, 2015), although there is no available literature on anti-angiogenic property of aqueous extract. In present study, it could not be concluded, whether the anti-angiogenic property of biogenic silver nanoparticles is solely due to nanoparticles or some effect is attributed to neem extract also and it is a further area of investigation. During green synthesis of nanoparticles, proteins present in extract acts as stabilizer upon binding with free amine or cysteine group. Carbonyl group from amino acids of protein has higher affinity for metal, and therefore surrounds the nanoparicle completely forming a layer and thus prevents agglomeration and stabilizes the nanoparticles. Agglomeration is the phenomenon, explaining the property of nanoparticles, where, due to high diffusivity nanoparticles exist as individual particles for only a short time and will agglomerate rapidly (Wong et al., 2009).
In conclusion, the angiogenesis reductive property of silver nanoparticles as revealed in the present study in chicken embryo model can have therapeutic value against pathological conditions, pathological angiogenesis, and especially to treat solid tumors, where abnormal and excessive angiogenesis is the cause of tumor growth. Further explorative anti-angiogenesis studies with the use of silver nanoparticles produced by green technology are suggested in the direction to treat disease conditions with pathological angiogenesis and address tumor growth treatment avenues.
Conflicts of Interest
The authors declare that no conflict of interest.
ACKNOWLEDGEMENTS
All the authors of the manuscript thank and acknowledge their respective Universities and Institutes.
Author’s contribution
Rekha Khandia and Ashok Munjal designed study and interpreted the results. Raj Sonam Bangrey and Reena Mehra collected review and updated article. Kuldeep Dhama and Naresh Chandra Sharma reviewed and editted the manuscript.
REFERENCES