Advances in Animal and Veterinary Sciences
Research Article
Sumbawa Mare Milk as a Preventive against Inflammation in the Gaster of Inflammatory Bowel Disease (IBD) Animal Model
Nurina Titisari1*, Tiara Widyaputri2, Ristia Mahfuzah3, Edwin Widodo4
1Department of Physiology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 3Student, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; 4Departement Of Physiology, Faculty of Medicine, Brawijaya University, Malang, Indonesia.
Abstract | Inflammatory Bowel Disease (IBD) is one of the chronic inflammatory diseases that occur in the digestive tract and often attacks older animals. Prolonged inflammation may cause digestive organ damage which is characterized by an increase in proinflammatory cytokines. This study was conducted to determine the preventive effect of Sumbawa mare milk on TNF-α level and the number of inflammatory cells in the gastric organs of IBD induced by indomethacin model animal. 20 rats were divided into 5 treatment groups, namely the negative control group (K-), the positive control group (K +) and the preventive group of Sumbawa mare milk with a volume of 0.5 mL / rat (T1), 1 mL / rat (T2) , and 1.5 mL / rat (T3) for 21 days. Groups K +, T1, T2, T3 were induced with indomethacin single dose (dose: 15 mg / kgBW) to make IBD model animal. The parameters observed in this study were TNF-α expression using the Flowcytometry method and the number of inflammatory cells counted in 5 gaster histopathic field of view. Data analysis of the study results was carried out using one way ANOVA (Analysis of Variance) method with a 95% confidence level (p <0.05) and continued with Tukey test. The results showed a decrease in Inflammatory cell numbers in gastric organs in all groups of preventive groups (T1, T2, T3) compared to the positive control group. The T3 group was not significantly different from the K- group. This is in line with the decrease in TNF-α level in all preventive groups (T1, T2, T3) which are significantly different from the K + group but not different from the K- group. Conclusions of this study indicate the administration of Sumbawa mare milk in animal models can prevent changes in gastric histopathology, reduce inflammation cells infiltration and reduce TNF-α expression The best volume of administration in this study was 1.5 ml / rat per day.
Keywords | Indomethacin, Inflammation cells, Gaster, mare milk, TNF-α
Received | April 22, 2020; Accepted | August 15, 2020; Published | September 01, 2020
*Correspondence | Nurina Titisari, Department of Physiology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia; Email: [email protected]
Citation | Titisari N, Widyaputri T, Mahfuzah R, Widodo E (2020). Sumbawa mare milk as a preventive against inflammation in the gaster of inflammatory bowel disease (IBD) animal model. Adv. Anim. Vet. Sci. 8(11): 1170-1174.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.11.1170.1174
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Titisari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Inflammatory Bowel Disease (IBD) is a chronic inflammatory disease that occurs in the digestive tract and it is mediated by the immune system (Djojoningrat, 2006). A common symptom in animals with IBD is continuous weight loss and diarrhea (Kathrani et al., 2011). IBD can be treated using effective steroid drugs to reduce inflammation. But the long-term side effects of steroid drugs are inhibition of new bone formation and acceleration of bone damage (Waljee et al., 2016).
The cause of this disease is still not known with certainty. Recent findings indicate other causes such as hypersensitivity to antigens in the lumen or intestinal mucosa (Defarges, 2016). Not only with drug therapy, research using probiotics and prebiotics to treat IBD has been widely carried out. Probiotics can alter the flora of the gastrointestinal tract by competitive mechanisms, produce antimicrobial substances, or affect local immune responses. Interaction of probiotics with epithelial cells can accelerate the healing of inflammatory processes. The effects of probiotics can be enhanced by administering prebiotics which can stimulate the growth of probiotics (Hyams, 2004).
The Sumbawa mare milk contained a lot of Lactic Acid Bacteria (LAB) which is one of the probiotics. In addition, the bioactive compounds in mare milk can have a function as an antioxidants, anti-inflammatory, antimicrobial, antihypertensive, anticholestrol, cytomodulator and immunomodulator (Yuniati and Sahara, 2012). Sumbawa mare milk contains lysozyme (BM 17 kDa) and lactoferrin (BM 75 kDa) which can be used as antimicrobials (Saputro, 2016) and contain amino acid histidine primary structure as an antioxidant that protects the body from oxidative stress due to the inflammation process (Elias et al., 2008). So, the purpose of this study was to determine the potential of Sumbawa mare milk in preventing increased inflammation in the gaster by analyzing the number of inflammatory cells and levels of TNF-α as one of the pro-inflammatory cytokines.
MATERIALS AND METHODS
The test subject was 20 rats (Rattus norvegicus) strain Wistar with following category; male, 5-8 months old and 150-250 grams in weight. Rats were divided into five treatment groups namely negative control (K-) without treatment, positive control (K+) with indomethacin induction orally at a dose of 15 mg / kg BW, preventive group that was given the Sumbawa mare milk with doses of 0.5 mL (T1), 1 mL (T2), 1.5 mL (T3) for 21 days. On day 15, the Sumbawa mare milk group (T1, T2, T3) and positive control group (K +) induced with a single indomethacin at a dose of 15 mg / kg body weight. Euthanasia was carried out on the 22nd day. The use of experimental animals has passed the ethical clearance certificate of the Brawijaya University Research Ethics Commission (KEP-UB) No 1046-KEP-UB.
Gastric pyloric was divided into two parts. For histopathic preparation, the sample was put into 10% formalin solution and then stained with Hematoxylin Eosin.
Data analysis
Histopathology samples were observed using the Olympus BX51 microscope and then observed the number of inflammatory cells in 5 fields of view with 400x magnification. For TNF-α expression examination, it was performed using the flowcytometry method. All data obtained in a quantitative analysis using one way ANOVA (Analysis of Variance) test with a confidence interval of 95% (p<0.05) and then followed by the Tukey test if there are differences.
RESULT and DISCUSSION
Based on observations, the rat condition in 48 hours after indomethacin induction experiencing clinical symptoms such as diarrhea and weight loss. Diarrhea occurs due to absorption disorders in the stomach as an effects of indomethacin administration. Indomethacin inhibits COX-1 which plays a role in the formation of prostaglandins. The reduction of prostaglandin productions will lower the protection in the gastric mucosa. This condition will trigger inflammation and causing damage to the gastric mucosa (Takeuchi et al., 2003). Weight loss in rat happened due to the decreased appetite because of the pain in the rat abdomen.
Gastric organ microscopic observation
Gastric organ tissue normally consists of tunica mucosa, sub mucosa, muscularis, and serosa (Tortora and Bryan, 2009). The microscopic histopathological observations of the negative control group (K-) gastric tissue (Figure 1A) showed a normal gastric image in the presence of a clear inline cylindrical epithelium. The formation and the boundary between the gastric pit in the gastric mucosa are clearly visible, regular and do not experience erosion. There is no visible inflammation and pathological conditions. Whereas in the positive control group (K +) gastric organ tissue (Figure 1B) showed the presence of gastric mucosal epithelial erosion and inflammatory cell infiltration. In some parts of the villi lamina propia also found a mass of erythrocyte cells that indicate hemorrhage as a sign of inflammation.
Histopathological results of the mare milk treatment group showed that in all treatment groups there was an improvement in epithelial cell erosion and there was a visible reduction in inflammatory cell infiltration compared to the positive control group (K +) (Figure 1C, 1D, 1E)
Tissue damage in the gastric organs mucosa from histopathological examination in positive control group is the effect of indomethacin with a dose of 15 mg/kgBB per oral in rats. According to Hatazawa et al. (2006), indomethacin can inhibit two COX isoforms, which is COX-1 and COX-2 resulting in impaired gastric mucosal protection due to decreased gastric mucus production and increased gastric HCL production. Therefore, the gastric mucosa was damaged due to the acidic environment in the stomach accompanied by a decrease in the gastric mucosal barrier, so the manifestations are inflammation and inflammatory cell infiltration (Yadav et al., 2012).
Table 1: Mean value of inflammatory cell in gastric organ of IBD animal model showed the number of inflammatory cells decreases as the volume administration of Sumbawa mare milk is increased.
| Group | Inflammatory cell number mean ± SD (Cell/Field of view) |
| K- (negative control) |
7.00 ± 0.63a |
| K+ (positif control) |
15.6 ± 0.96d |
| T1 (0.5 mL Sumbawa mare milk treatment) |
11.7 ± 0.68c |
| T2 (1 mL Sumbawa mare milk treatment) |
10.05 ± 1.20bc |
| T3 (1.5 mL Sumbawa mare milk treatment) |
8.60 ± 1.79 ab |
Note: a, b, c and d notation showed that there is a significant difference (p<0,05).
Table 2: Mean value of TNF-α expression in the gastric organ IBD animal Model indicated a decline of TNF-α level expression in the mare milk treatment group compared to the positive group (K +).
| Group |
Mean value of TNF-α expression ± SD (% cells) |
| K- (negative control) |
31.89 ± 2.18a |
| K+ (positive control) |
59.99 ± 3.68c |
| T1 (0.5 mL Sumbawa mare milk treatment) |
45.00 ± 4.47b |
| T2 (1 mL Sumbawa mare milk treatment) |
43.93 ± 5.25b |
| T3 (1.5 mL Sumbawa mare milk treatment) |
43.38 ± 3.68b |
Note: a,b and c notation showed that there is a significant difference (p<0,05).
Inflammatory cells numbers in the histopathology of gastric organ on IBD animal model
Inflammatory cells observation in gastric histopathology in all treatment groups used a 400x magnification. The statistical results indicate a significant difference (p <0.05). The mean of inflammation cell infiltration number in the negative control group (K-) (7.00 ± 0.63a cells / field of view) showed the lowest mean number of inflammatory cells, whereas in the positive control group (K+) it showed the highest number of inflammatory cells (15.6 ± 0.96c cells / field of view) compared to other treatment groups.
Inflammatory cells that appear in the observation are mostly dominated by macrophages, lymphocytes and neutrophils. Neutrophils are inflammatory cells in the non-specific immune system that comes first when an inflammatory response occurs. On the other hand, lymphocyte cells are natural defenses in the organ of digestion, originating from the GALT (Gut Associated Lymphoid Tissue) system which is located in the basal zone of the gastric mucosa lamina propria (Janeway et al., 2001). The infiltration of inflammatory cells is caused by pro inflammatory cytokine activity. Macrophages produce and secrete TNF-α, a pro inflammatory cytokine as an indicator of inflammation, and TNF-α will activate from neutrophils to areas of inflammation (Takeuchi et al., 2003).
In the T1 (11.7 ± 0.68bc cells / field of view), T2 (10.05 ± 1.20b cells / field of view), and T3 (8.60 ± 1.79 ab cells / field of view) groups showed that there is a reduction in inflammatory cells numbers compared to the K + group (positive control). Furthermore, there was also gastric epithelium cells repair in the treatment group. The T3 group was not significantly different from the negative control group (K-) showing that a dose of 1.5 mL of mare milk per oral was able to effectively prevent an increase in the number of inflammatory cells in gastric organ of the rat.
The lysozyme that contained in Sumbawa horse milk is able to limit the migration of neutrophils to the damaged tissue and act as an anti-inflammatory agent. Based on its ability to bind Fe, lactoferrin has an important role in binding iron in the intestinal mucosa and acts as a bacteriostatic agent by binding to iron derived from iron needed for bacterial growth (Sacharczuk et al., 2005). Its presence in neutrophils and their releases during inflammation, strengthen the assumption that lactoferrin also plays a role in phagocyte destruction and immunity (Yuniati and Sahara, 2012). Lactoferin can induce mediators from innate immune cell that eventually can affect adaptive immune cell function. As a main immune homeostasis that play a role in reducing oxidative stress at the molecular level, therefore it can control the excess of inflammatory response (Actor et al., 2009).
TNF-α expression in the histopathology of the gastric rats IBD animal model
One way ANOVA results showed significant differences (p <0.05). Significant differences prove that the administration of sumbawa mare milk can prevent an increase in TNF-α levels (Table 2). The results showed that the highest mean value of TNF-α was in the positive control, the lowest was in the negative control, and there was a decrease in each group of mare milk treatment when compared to the positive control.
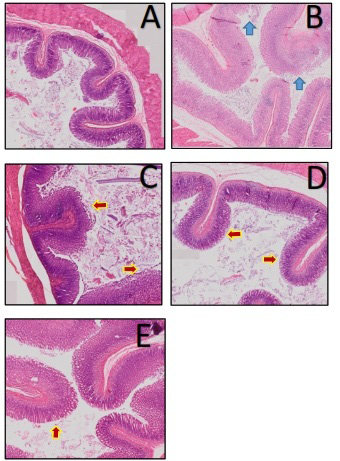
Figure 1: Histopathologic of rat gastric organ (Rattus norvegicus) Inflammatory Bowel Disease. Negative control (A) without treatment; position control indomethacin induction 15 mg/ kg (B); Sumbawa mare milk treatment 0.5mL (C); 1 mL (D); 1.5 mL (E). (HE staining; 100x magnification).
Note: (): gastric epithelial erosion; (): gastric epithelial repair.
The positive control group (K +) showed the highest mean value of TNF-α expression compared to the other treatment groups (59.99 ± 3.68c). An increase in TNF-α indicates an inflammation process occur in the rat gastric organ. Indomethacin is lipophilic and binds to cell membranes composed of phospholipids which will increase the formation of ROS and activates NF-kB (Nuclear Factor kB). Increased NF-kB activation will be responded by macrophages to produce and secrete proinflammatory cytokines TNF-α as indicators of inflammation, and TNF-α will activate from neutrophils to areas of inflammation (Takeuchi et al., 2003).
In all of the mare milk treatment groups, there was a decrease in TNF-α expression compared to the positive control group (K +) (Table 2). T1, T2 and T3 groups were not significantly different. This means that the administration of mare milk with a dose of at least 0.5 mL (T1) can reduce the amount of TNF-α pro inflammatory cytokines in the rat gastric organ. Mare milk affect the modulation of the inflammatory process through its effect on the reduction of the inflammatory cell chemotaxis process and respiratory burst (rapid increase in ROS of various cell types), so that it can treat recurrent inflammation (Ellinger et al., 2002).
Lactoferrin has the ability to increase phagocytosis and cell adherence and control the release of proinflammatory cytokines. It is also can reduce apoptosis induced by oxidative stress because of its ability as an antioxidant (Actor et al., 2009). In addition, probiotics found in Sumbawa mare milk can improve the function of immunologic barrier of intestinal mucosa through excretion of pathogenic microbial, restore the intestinal mucosal surface permeability to normal condition in allergic or inflammatory diseases, and reduce proinflammatory cytokine products (Diding et al., 2008).
CONCLUSION
Based on this research, it was found that Sumbawa mare milk as a preventative in rat (Rattus norvegicus) IBD model could repair the histopathological image of the gastric, less inflammatory cells infiltration and TNF-α expression compared to the positive control group. The best preventive dose is treatment group 3 with Sumbawa mare milk volume of 1.5 mL.
ACKNOWLEDGEMENTS
The present scientific research was financially afforded by Faculty of Veterinary Medicine Research Fund, 2019, Brawijaya University, Malang, Indonesia
AUTHORS CONTRIBUTION
The authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES






