Advances in Animal and Veterinary Sciences
Review Article
Bone Tissue Engineering: Latest Trends and Future Perspectives
Rekha Pathak1*, Amarpal1, Hari Prashad Aithal1, Prakash Kinjavdekar1, Abhijet M Pawde1, Rashmi1, Tamil Mahan1, Netrapal Sharma1, Kuldeep Dhama2
1 Division of Surgery, 2Division of Pathology, Indian Veterinary Research Institute, Izatnagar-243122, Bareilly, India.
Abstract | Bone heals without any scar formation and provides the structural support to the body. The bone gap defects / loss of bone created during trauma, resections and neoplastic/ diseased bone removal can be treated by implanting the bone grafts in the defect site. These grafts can be synthetic or of biological origin. The synthetic grafts take long time for the resorption. Due to the microstructural similarity of the biological as origin grafts with the host tissue they are thought to be ideal grafts. Autograft is a gold but certain disadvantages like antigenicity, immune rejections and pathogen transmission are the associated with allograft and xenografts. Antigenicity can be reduced by processing the tissues for the removal of the cells and rendering them acellular. It is usually the cellular component which elicits the host immune response and brings about rejection of the grafts. Various processes employed for the decellularization are snap freeze thaw technique, chemical treatment like acetone -ethanol processing and sodium do-decyl sulphate. Rapid freeze and thaw technique can be recommended strongly for the bone decellularization which causes minimum damage to the extracellular matrix (ECM) and also brings about an ideal integration with the host tissue. The acellular bone grafts can be enriched with osteoblast cells to increase the regenerative capacity of the bone. The fetal osteoblasts have been found to be immune privileged and also aid in acceptance of the graft. The grafts can also be rendered more osteoinductive by adding a component of the growth factor in the scaffold composite. The uses and disadvantages of allograft, autograft and xenograft have also been discussed. It is also necessary that these biomaterials/ implants should be ready to use kind of stuff so that there is minimum time paucity after the case is presented with complicated gap defects of bone. There is a tremendous scope of bone tissue engineering for the orthopaedic surgeons. This opens a positive solution for the problems like loss of bone and limb amputation.
Keywords | Tissue Engineering, Bone, Scaffolds, Osteoblast, Bone grafts, Segmental bone defects
Editor | Muhammad Munir (DVM, PhD), Avian Viral Diseases Program, Compton Laboratory, Newbury, Berkshire, RG20 7NN, United Kingdom.
Special Issue | 4(2015) “Advances in animal disease diagnosis, vaccines and therapeutics”
Received | January 18, 2015; Revised | February 20, 2015; Accepted | February 22, 2015; Published | March 04, 2015
*Correspondence | Rekha Pathak, Division of Surgery, Indian Veterinary Research Institute, Izatnagar, Bareilly, India; Email: [email protected]
Citation | Pathak R, Amarpal, Aithal HP, Kinjavdekar P, Pawde AM, Rashmi, Mahan T, Sharma N, Dhama K (2015). Bone tissue engineering: latest trends and future perspectives. Adv. Anim. Vet. Sci. 3(4s): 9-22.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.4s.9.22
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Pathak et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Among all the transplants made, bone is said to be the second most commonly transplanted tissue after blood (Nandi et al., 2010). The loss of bone due to damage is a very challenging problem for the orthopaedic surgeons especially in those cases where loss of bone is massive. It is a well-known fact that autografts serve as the gold standard for bone grafts because apart from the ideal compatibility with the tissue from the structural and immunological view point they also offer all the desirable characteristics of the bone graft material. They are rich in osteoinductive (bone morphogenic protein), osteogenic (MSC/osteoblast cells) material and it serve as a best osteoconductive (three dimensional and porous) material. It requires a site of harvest of the tissue which not only increases the pain but also increase time of surgery, bleeding and donor site morbidity (Younger and Chapman, 1989; John et al., 2003). It also leads to nerve injuries at the multiple harvest sites due to the surgery (Ehrler and Vaccaro, 2000). The areas which require a large amount of tissue cannot be transplanted with the autograft as the amount of collected tissue is very limited. The allograft and xenograft in this situation can be thought of as better option in terms of availability and structural similarity with the host. Bone tissue engineering involves combination of innovations made by different disciplines of science (Marco et al., 2014). Grafts that are well tolerated, resist infections and rapidly vascularised are the ideal characteristics of a biologic graft (Jacobsen and Easter, 2008). Allograft and xenograft are associated with the surface antigens and other proteins which sometimes lead to rejection and necrosis. In such situations they require an in vitro chemical processing which brings about removal of all the antigenic materials which can be quantified indirectly by protein estimation and total DNA estimation (Zhang et al., 2009). There are a number of commercially available bone graft substitutes available as solid grafts wedges, granules, DBM (Demineralized bone matrix) and putty etc., which serve as a very positive solution for the massive orthopaedic injuries involving reconstruction in human beings and animals. The osteoconduction and biodegradable quality makes them the excellent bone grafts (Oryan et al., 2014). However, in the expanding scenario of tissue engineering still a considerable validation regarding the host immune response with the biological grafts are needed.
Bone grafting
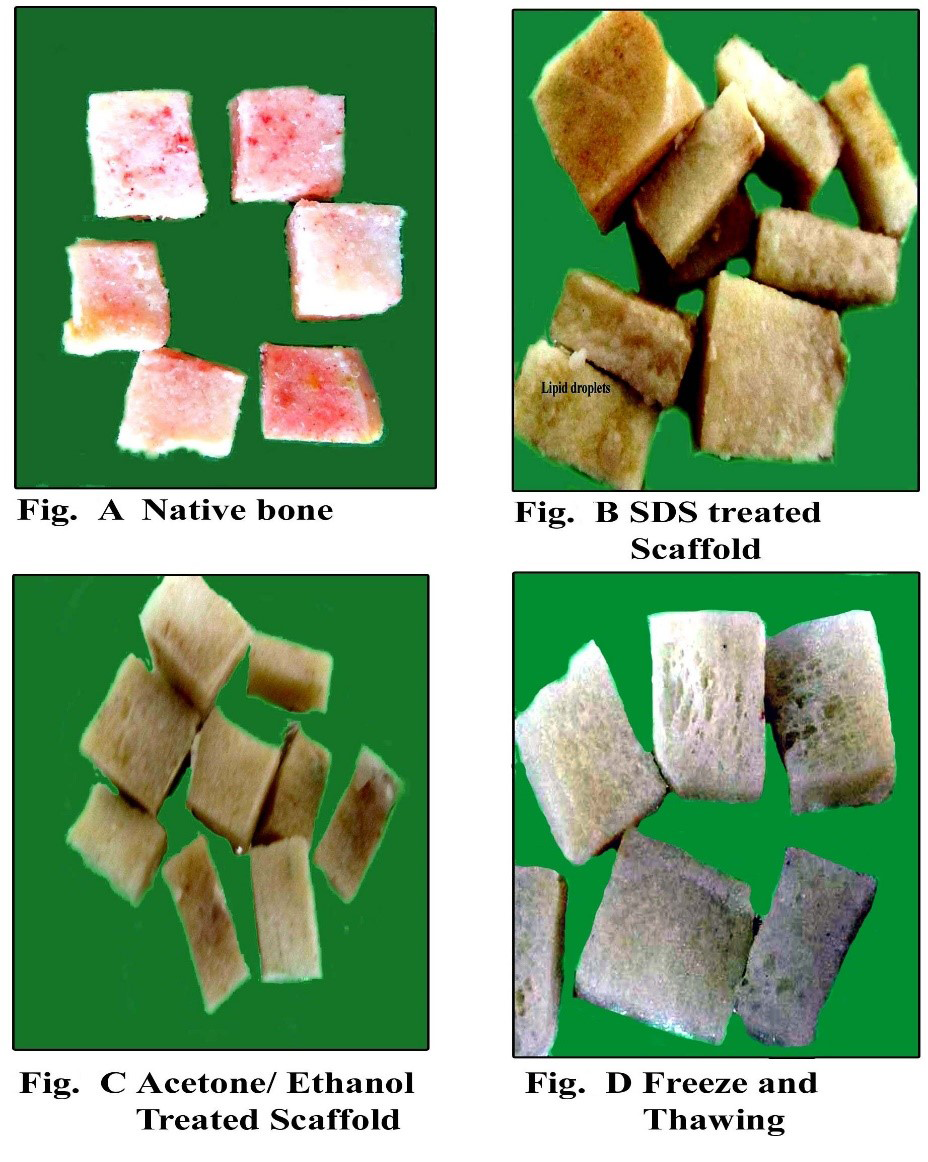
Bone is highly dynamic and vascularised tissue which heals without any scar formation (Sommerfeldt and Rubin, 2001). Its main role is to provide structural support for the body. Although there is a tremendous healing capacity of bone but it is compromised to larger extent when there is a big amount of bone such as, in cases of severe trauma, developmental deformities, osteolytic disease, and tumour resection (Perka et al., 2000; Gugala and Gogolewski, 2002). Autograft harvesting causes the donor site morbidity and limited amount of tissue harvest (Seiler and Johnson, 2000; Antonio et al., 2004) which limit the usage of autograft. The allografts are associated with immune rejection and pathogen transmission (Berrey et al., 1990; Lucarelli et al., 2005). There are many synthetic materials which can be used to fill bone defects in experimental and clinical animal studies include growth factor, calcium phosphate, hydroxyapatite, tricalcium phosphate, type I collagen, bioactive glasses, and synthetic polymers (Shand and Heggie, 2000; Finkemeier, 2002; Hing et al., 2006). Imperfect osteoclastic resorption and remodelling are the critical disadvantages in the clinical use of synthetic scaffold are (Aro et al., 1983; Trentz et al., 2001). Tissue engineering employs the knowledge of engineering in life sciences to restore the tissue function by the use of biological substitutes (DeCoppi et al., 2007). Xenogenic bone grafts are preferred due to its unlimited supply especially when collected from a larger species like bovine. However, the limiting factor in the usage of xenogenic grafts are the presence of xenogenic antigens and rejection of the graft sometimes. It therefore becomes necessary to remove these antigens to minimize or avoid the immune response responsible for non-acceptance/ rejection (Erdag and Morgan, 2004; Gock et al., 2004). To accomplish this feature decellularization is widely adopted technique and is resulted in unaltered biologic activity and mechanical integrity/ strength (Badylak, 2004; Gock et al., 2004). It is said that only extracellular matrix mainly collagen is left after the decellularization process and are largely and highly conserved across species lines (Constantinou and Jimenez, 1991; Exposito et al., 1992). Various physical, chemical, enzymatic digestion methods are employed to render the tissue of biological origin acellular. Usually the preference is to accompolish complete decellularization with minimum alteration in the tissue architecture. Decellularization cause sometimes loss in glycosaminoglycans (GAG) and also the alteration in the strength of the material which is very crucial in case of bone. The staining for GAG is done to correlate the loss of GAG with the intensity of the stain when different decellularization techniques are employed (Figure 1).
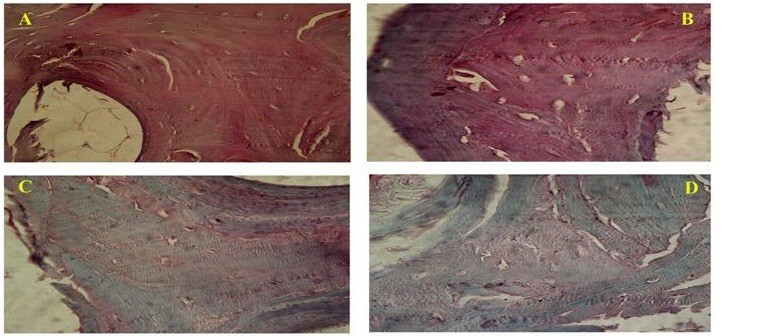
Figure 1: Photomicrograph showing (A-Native bone) intense GAG staining, (B-SDS treated bone) preserved GAG, (C-Acetone/Ethanol treated) less staining for GAG and (D-Freeze and Thawing group) less GAG content as compared to native bone. (Safranin-O; x40)
Mostly the scaffolds used in tissue engineering are the naturally occurring ones. Many of these scaffolds are completely biodegradable and are replaced by new host tissue. The scaffolds are also sometimes cross linked by chemicals in order to achieve the required strength and resist the fibroblastic invasion and degradation (Badylak, 2007). The non-cross linked scaffolds are said to be replaced readily by neoconnective tissue formation (Trabuco et al., 2007) but for the tissues like bone a material which has a long term stay at the site with ability to pace with the rate of substitution by the host tissue is required.
SDS is an ionic detergent (Chen et al., 2004; Hudson et al., 2004) that has recently been used to decellularize the bovine trabecular bone (Grayson et al., 2008; Frohlich et al., 2010) and bovine intervertebral disc (Chan et al., 2013). The bovine matrix is the natural biomaterial obtained from bovine cancellous bone. It is found that the Cancellous bone grafts get integrated with the host faster in terms of revascularization than the cortical grafts (Pinholt and Solheim, 1994; Stevenson et al., 1996). It is so because the permeability to blood vessels is easily rendered by cancellous bone and it also allows rapid movement of the required nutrients from the host tissue. This probably leads to increase in the bone cell survival which later on deposit the osteioid tissue in the spaces of trabecular xenograft. The rate of degradation depends upon the method of decellularization and origin of tissue (Gilbert et al., 2006). There have also been studies where the bovine bone scaffolds prepared by different decellularization techniques are compared biomechanically, clinically and immunologically scaffolds prepared by freeze and thaw cycles when compared to Acetone ethanol processing and SDS 1% processing was found to be superior both in vitro and in vivo for bone tissue engineering in rabbits. The tensile strength was unaffected and the antigenicity was also reported to be minimized (Tamilmahan, 2013).The freeze and thaw treated tissues looked brighter and whiter than other treatments (Figure 2).
Biodynamic of bone
Bone is an organized tissue with mineralization and helps not only in providing the skeletal support but also contribute in the calcium homeostasis (Jee, 2001). Trabecular bone is a porous structure having around 50-90% porosity. The bone marrow is rich in stem cells which is essential in regeneratin of tissues (e.g. muscle, cartilage, bone, and tendons) and also produces haematopoietic cells. The Cortical bone on the other hand is very hard and compact with very less porosity of only 10% (Jee, 2001).
The osteoblasts give rise to the osteocytes which form the integral part of bone lining (Calvi et al., 2003; Zhang et al., 2003). Osteoblasts cells are typically rounded, polyhedral and flat in their morphology having a diameter of around 20 mm (Jager et al., 2007; Pathak et al., 2012). These cells synthesize the osteoid containing type I collagen, osteocalcin, (Pathak et al., 2012) osteopontin, bone sialoproteins, and bone morphogenetic proteins (Robey and Termine, 1985). Angiogenesis is stimulated by certain substances secreted by the osteoblasts (Fuchs et al., 2007; Santos et al., 2009). The remodelling of bone is brought about by the Osteoclasts which help in resorption of the bone at the tension site. When the bone is subjected to natural pressures of load, there is an orientation and parallel arrangement of the fibres making the bone more strongly defined in terms of strength. The cellular functions are ultimately enhanced or inhibited according to the body requirement. Figure 3 is showing the different types of bone cells derived from the mesenchymal bone marrow cells on the surface of bone. It is importantly noted that the osteoblast cells migrate on the concave surface and osteoclasts act on the tension site/ convex surface of the bone thereby bringing a proper remodelling.
Bone healing
The natural fracture healing process occurs in three principal phases. The inflammatory phase occurs first and then is followed 4 to 5 days later by the repair phase during which mesenchymal cells invade the fracture location and subsequently differentiate into fibroblasts, chondroblasts, and osteoblasts. This event represents the formation of a soft callus, which has the ability to stabilize the fracture site, thus allowing mineralization to occur and strengthen the repair (McKibbin, 1978). The completion of the healing process is marked by the remodelling phase in which the callus remodels to woven and then lamellar bone. The recruitment of several cell populations, including fibroblasts, macrophages, chondroblasts, osteoclasts, and osteoblasts influence the repair process of fractured bone. Usually the presence of osteoconductive surface, osteoinductive factors, immobilization of the fractured site and osteogenic cells lead to a successful fracture healing (Giannoudis et al., 2007). However sometimes there is a serious compromise in the healing / reparative ability of the bone. In the various conditions like osteogenesis imperfect, large critical sized defects, infections, radiation therapy etc this regenerative ability of bone is hindered (Kamboj, 2007; Mehta et al., 2010). Tissue engineering has is a potential solution to overcome these difficult situations. It not only provides a conducive environment for the healing of tissues but also focuses on the regenerative aspects.
Type of Bone grafts
Bone graft matrix and synthetic osteoconductive polymers are the osteoconductive materials. A mechanical substrate over which cells anchor and along which cells proliferate to form the new bone serves as a scaffold. The osteoblasts and growth factors serve as osteogenic component of bone formation (Perry, 1999).
Bone grafting in human dates back to 1668 where Job van Meekeren; a Dutch surgeon corrected skull defect in a Russian nobleman by using piece of canine skull xenograft. The patient was abandoned by the Christian society (Boer, 1988). Louis Xavier Eduard Leopold Ollier (1830-1900) suggested that the epiphyseal plate resection and cartilaginous stimulation can inhibit the bone growth Sir William Macewen (1848-1924) developed a one-piece osteotome. Sir Robert Jones (1855-1933) performed tendon transplantation, bone grafting and restoratie procedures. The various classification of bone grafts are autograft, allografts, xenografts and synthetic grafts (Bauer and Muschler, 2000).
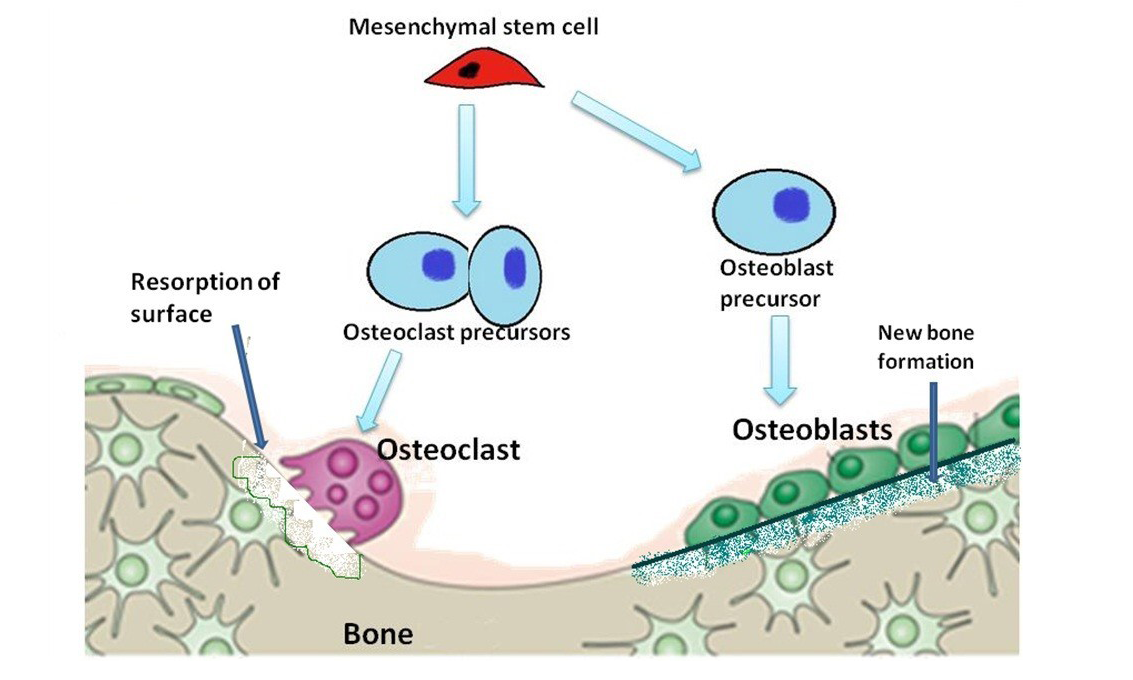
Figure 3: Diagram is showing evolution of osteoblasts and osteoclasts in the formation and resorption of bone
Autografts are considered to be gold standards in bone grafting due to their best compatibility and easy acceptance by the host tissue (Samartzis et al., 2005). The cortical grafts which are vascularized are generally superior over the non-vascularized in terms of faster healing and remodelling and they provide superior strength during the first six weeks.
The allogeneic grafts present osteoinduction and osteoconduction abilities, being processed in sterile conditions and stored in bone banks (Merkx et al., 2003; Murugan and Ramakrishna, 2005). These grafts are not associated with donor site morbidity and unlimited amounts of graft material can be collected. Their main disadvantages include mostly risk of transmission of infectious diseases, host immune response and lower predictability for the grafting outcome. Various physical processing like freezing, freeze-drying, or irradiation of the grafts reduces the antigenicity. Allograft in equine fractures have been reported. However, they take a long time for incorporation.
During the freeze drying the moisture gets removed and the mechanical strength of the bone is greatly reduced (Marx, 1993). The osteoinductive property is lacking in such grafts and hence freeze-dried allogeneic implants are usually placed in conjunction with autogenic grafts when reconstructing the craniomaxillofacial skeleton. Demineralized bone matrix (DBM) retains the inductive properties but becomes weaker in strength (Zhang et al., 1997). Removal of the mineral component from the bone matrix may expose native proteins, such as bone morphogenetic protein (BMP).
Xenograft bone or collagen has been experimentally explored as a bone substitute (Marchesi, 2000; El-Sabban et al., 2007; Stievano et al., 2008). The donor site morbidity is not the constraint at all an additionally these grafts are also ready to use and commercially available. The processing aimed at removal of cells and proteins reduced the antigenicity of these grafts (Iwamoto et al., 1997) and increase the host acceptance (Basle et al., 1998).
It is highly desirable that antigenic epitopes should be removed by decellularization protocols. Physical methods, chemical detergents and enzymatic solutions can effectively remove the antigenic epitopes, cellular and nuclear material. The adverse host reaction and graft rejection can thus be avoided. The ideal decellularization method preserves the native ultra-structure and composition of extra cellular matrix (ECM) while removal of cellular material (Ott et al., 2008; Grayson et al., 2008; Uygun et al., 2010).
Physical Methods of Decellularization
Freeze-thaw cycles, direct pressure, sonication, and agitation of the tissues are few methods by which the bone is rendered acellular. The tiny ice crystals formed inside the cell brings about the rupture of the cell memberane and cells lysis. There should also be certain processes developed to remove the cellular material from the tissue which is frozen. Enzymatic treatment followed by mechanical abrasion (force) helps removal of cells on the surface of a tissue or organ (e.g., urinary bladder, small intestine, skin, and amnion) (Hopkinson et al., 2008). However, underlying ultra-structure and basement membrane integrity are invariably damaged by any direct application of mechanical force (Hopkinson et al., 2008). The chemical treatment: when the tissue is subjected to the chemical processing with acid and alkali, the cellular components are dissolved and nucleic acids are also removed (Falke et al., 2003; Freytes et al., 2004). Hypertonic and hypotonic solutions also help rinse cell residue from within tissue following lysis. Treatment of cartilage with hypotonic and hypertonic solution showed slight reduction in number of cell nuclei and reduce GAG content (Elder et al., 2009). It is also important that these agents should be completely removed from the ECM (Cebotari et al., 2010).
Sodium dodecyl sulfate (SDS) is used for the decellularization of dense tissue and preserves the tissue strength also (Lumpkins et al., 2008; Nakayama et al., 2010). The main disadvantage is growth factors are also eliminated (Reing et al., 2010). The tensile strength and the compressive modulus were observed to be significantly decreased (Tamilmahan, 2013).
Triton X100 when tried on the swine tempero mandibular joint (TMJ), revealed decreased the mechanical integrity and inferior energy dissipating capabilities of the TMJ to that of the native tissue. Another protocol included a 1% SDS treatment which not only preserved the size of the discs but also the modulus values were preserved after decellularization and there was no significantly difference between the native tissue and SDS treated tissue (Lumpkins et al., 2008).
Other solvents like alcohols such as glycerol render tissue decellularization by dehydrating and lysing cells (Prasertsung et al., 2008). The alcohols like methanol and ethanol are more effective than lipase but also fixes the tissue and proteins (Jamura and Oliver, 2010). It is also found that ECM ultrastructure is not preserved (Gorschewsky et al., 2005). The lipid removal is aided by Acetone in tissue processingo (Lumpkins et al., 2008; Montoya and McFetridge, 2009). It leads to crosslinking of ECM and increases the strength (Lumpkins et al., 2008). Acetone treated bovine cancellous bone rendered, shortened and flattened collagen bundles than native bone and bone became more brittle (Pathak et al., 2012).
Tissue treatment with trypsin and DNase and RNase also brings about the nucleotides removal and better preservation of GAG (Petersen et al., 2010; Grauss et al., 2005). The chelating agents such as EDTA, acts by binding the divalent cations that are present at the cell adhesions to the ECM, and facilitate removal of the cellular material from the tissue. But the Chelating agents alone are not sufficient for superficial cell removal even with agitation; the combination treatment with enzymes such as trypsin or detergents is also required (Hopkinson et al., 2008).
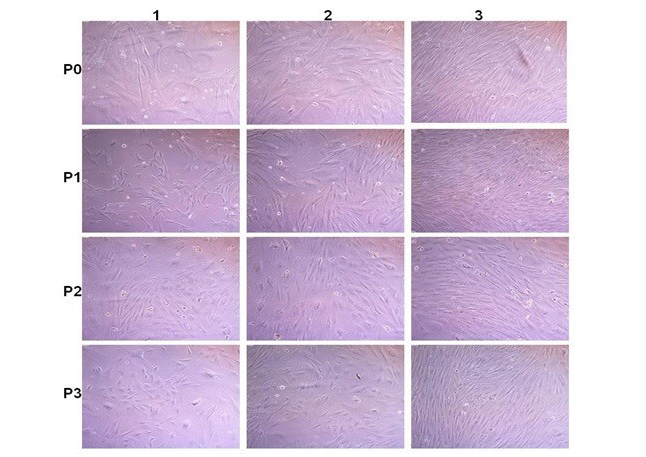
Figure 4: Rabbit fatal Osteoblast cell culture: P0; P1; P2 and P3 are passaged. 1; 2 and 3 images after 2; 6 and 8 days after passages. Cells become confluence (80-90% growth) within 6-8 days after passage
The source of tissue if allogenic/ xenogenic requires complete and effective decellularization. Similarly the thick and dense tissues like bone and tendons need a critical protocol of decellularization. The selection of technique is made by keeping in mind the mechanical strength of the tissue. It is aimed that a complete cellular removal with minimum damage to the ECM should occur. The host tissue response following in vivo implantation of these scaffold materials is dependent upon the efficacy of decellularization and removal of cell remnants. The main components of the ECM include collagens, glycosaminoglycans, non-collagenous structural proteins, and proteoglycans. The basement membrane is an important type of ECM that is composed of laminin, network-forming collagen type IV, nidogen, an perlecan. Collagens are responsible for the basic structure of the ECM, providing its structural integrity. Many of the fibrillar collagens, including types I, II, III, V, and XI, impart tensile strength (Bosman and Stamenkovic, 2003) in healthy tissues. Glycosaminoglycans (GAGs) are re- sponsible for imparting gel properties to the grafts and are also associated with growth factors and cell messenger interaction. So the GAG rich in heparin become more important due to the high affinity of cell surface receptors and many growth factors with heparin (Hodde et al., 1996). Signalling conducted through integrin-integrin interactions influences cell survival, proliferation, the structure and functional activity of the cytoskeleton, and gene transcription. Other ECM components include the cell-adhesive glycoprotein fibronectin, paracrine signalling molecules including TGF-β (hormone involved in cell growth and differentiation and the composition of the ECM), fibroblast growth factor (FGF), and other growth factors that mediate cell behaviour and function (Nelson and Bissell, 2006).
Composite Grafts and Seeding Protocol
The grafts that are seeded with the proliferative cells specific for the tissue implanted on are said to be the composite grafts. The disc seeded multiple bone graft was successfully applied in a 52 year old woman to treat the segmental bone defect (Hesse et al., 2010). Implantable three-dimensional (3D) living constructs were prepared by seeding isolated bone marrow stromal cells from the patient onto decellularized bovine trabecular bone scaffolds. There was no pain and swelling at the site. It took a total of 5 - 7 months for the complete fusion between the implant and the host bone posturgery. The proper nutrient supply and oxygen in the inner microenvironment of the scaffolds forms a basis to initiate scaffold substitution and to improve cell performance in tissue-engineered approaches to bone repair where ceramic biomaterial was seeded with mesenchymal progenitor cells (Giannoni et al., 2008). In one study of Liao and co-workers (2004) neonatal rat osteoblasts have changed the shape from spindle to polygonalAnd also the proliferation and adhesion was more on the porous surface of the implant.
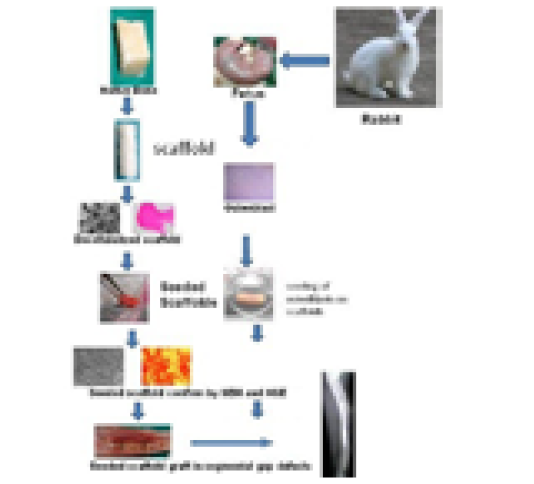
The in vitro culturing of cells is done to increase the effective population of cells which is required for tissue engineering. These cells are required to be seeded on the ECM. The seeding can be done by static or dyanamic methods. Static cell seeding doesnot require any movement in the flow of the medium through the scaffold (Datta et al., 2005). Dynamic conditions may offer certain advantages, in contrast to static conditions, since the cells are given more time and thereby opportunity to adhere, and thus may lead to a positive outcome through higher seeding efficiency. In our laboratory also the osteoblasts cells are cultured and expanded for the in vivo use/ seeding of the scaffold at a certain concentration/ density (Figure 4). The process of graft preparation and in vitro and in vivo evaluation host response to extracellular matrix Scaffolds Host Response to Extracellular Matrix Scaffolds is depicted in Figure 5.
The dyanamic system of seeding comprises of the dyanamic flow of the media around the constructs and encourages more penetration of the cells into the scaffold core. This can be achieved with the help of specially designed seeding bioreactors. Gas exchange is performed via surface aeration (Bancroft et al., 2003; Kitagawa et al., 2006; Vunjak-Novakovic et al., 2005).
Host Response to Extracellular Matrix Scaffolds Host Response to Extracellular Matrix Scaffolds
The Xenogeneic and allogeneic cellular antigens cause the host to release mediators of inflammation and transplant tissue rejection. However, the mechanism of rejection still remains obscure (Badylak and Gilbert, 2008). The ECM is usually said to be conserved across species and the side effects of the immune response can be eliminated (Allman et al., 2001). The adverse effects of the immune response is through the components that include the Galα1-3Galβ1-4GlcNAcβ-R (α-Gal) epitope and DNA. The α-Gal epitope is known to cause hyperacute rejection of organ transplants (Oriol et al., 1993; Collins et al., 1994). However, the ECM containing the α-Gal epitope were free of any adverse effects (Raeder et al., 2002; Daly et al., 2009).
The presence of nuclear material is also said to influence the immune response of the host. However many commercially available scaffolds reflects the presence of DNA in small quantities (Gilbert et al., 2009) .It is found that the remnant DNA fragments generally are less than 300 bp. Inspite of the presence of alpha Gal epitope and DNA fragments, the adverse response is not observed. This implies that probably a minimum effective amount of these components are necessary to elicit any side effect on the remodelling response. Neutrophils and macrophages are the first cells to face the implanted biomaterials. Initially neutrophil begin to invade at the site which in the absence of debris and endotoxins begin to disappear and mononuclear cell population replaces the cell type. It is said that these cellular events bring about either acute or chronic inflammation and are associated with negative effect on healing. However, the presence of these cells, especially mononuclear macrophages, has been shown to be essential to the formation of the type of constructive tissue remodelling response that has been observed following the implantation of ECM scaffolds (Badylak et al., 2008; Brown et al., 2008).
The T-lymphocyte response to xenogeneic muscle tissue, syngeneic muscle tissue, and an acellular ECM scaffold was examined by subcutaneous implantation. The xenogenic imlant was rejected with signs of necrosis, granuloma formation and encapsulation. There was an acute inflammatory response with syngeneic tissue and the ECM scaffold but it settled with time and an organized tissue was formed. Tissue cytokine analysis revealed that the ECM group elicited expression of interleukin (IL)-4 and suppressed the expression of interferon (IFN)-γ as compared to the xenogeneic tissue implant group. The ECM group elicited the production of an ECM specific antibody response, however it was restricted to the immunoglobulin G (IgG) 1 isotype. Re-implantation of the mice with another ECM scaffold led to a secondary anti-ECM antibody response that was also restricted to the IgG1 isotype and there was no evidence of the formation of a Th1 type response. Further investigation confirmed that the observed responses were in fact T-lymphocyte dependant. So it can be concluded that both types of cells T and B cells respond to ECM scaffolds, but do not influence the constructive tissue remodeling of an ECM implant (Allman et al., 2001).
Fate of the graft: Initial vacularization of the graft is extremely required for the graft to survive, integrate and function (Carano and Filvaroff, 2003). The cortical graft gets slowly vascularised as compared to the cancellous grafts and requires an initial phase of bone resorption. The cortical grafts begin to unite at the host graft junction first and healing gradually moves towards the shaft of the graft. There is the repair and remodelling of cortical bone occurs at a very slow rate, and any imbalance between resorption and bone formation can lead to bone loss and graft failure (Burchardt, 1987; Bauer and Muschler, 2000). Fibrotic nonunions and late graft fractures are reported to be directly dependent upon the neovascularization of structural allografts with 25% clinical failure rate (Berrey et al., 1990). In human studies it has been shown that 1-2 years after surgical implantation, the fractures occur and are related to initiation of microfractures within the dead cortical bone (Wheeler et al., 2001). In contrast, cancellous bone graft is porous revascularize quickly, and can be rapidly incorporated and remodelled. In this type of graft, osteoblastic bone formation occurs first on the surface of necrotic trabeculae followed by osteoclastic bone remodelling and gradually resorbtion of the entrapped dead trabeculae and eventually replaces the entire graft with new living bone (Rashmi, 2014).
It is required that the biomechanical strength of the bone implant should be more due to the load bearing nature of the bone (Vunjak-Novakovic and Goldstein, 2005).
Conclusion
It can be concluded that the scaffolds for bone should be ready to use kind of stuff so that there is minimum time paucity after the case is presented with complicated gap defects of bone. The bone bioengineering is an upcoming area which offers a viable solution for the problems like loss of bone and limb amputation. The combination of regenerative cells and ECM matrix not only provide a support at the site of loss but also provides the factors important for the bone regeneration and healing.
References