Advances in Animal and Veterinary Sciences
Research Article
Oxidative Stress Associated with Canine Leishmaniosis with Special Reference to Haemo-Biochemical Changes
Sabry A. Mousa1, Marwa M. Attia2*, Arafat Khalphallah3, Noha Y. Salem1
1Department of Internal medicine and infectious diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 3Division of Internal medicine ,Department of Animal Medicine , Faculty of Veterinary Medicine, Assiut University; Assiut, Egypt.
Abstract | The aim of this study is to identify the causative protozoan parasites infecting dogs in Egypt by discussing the clinical signs, hemato-biochemical, oxidative stress, and the pro-inflammatory markers alterations in Canine leishmaniosis (CanL). A total of 100 dogs of different breeds were collected from January to December 2020. The age of the dogs ranged between 3and 10 years. All the 100 dogs suffered from signs compatible with vector-borne diseases. Only one reported case was recorded for infection with CanL from 100 examined dogs (1% infection rate). The dog was 7 years old female German Sphered dog with significant weight loss, depression, pyrexia (temp=40º), lameness on hind limbs, severe respiratory manifestations, and skin ecchymosis in the ventral abdomen, tail, and hind leg with lymphadenopathy. It had marked leukocytosis, neutrophilia, and monocytosis. Normocytic normochromic anemia and thrombocytopenia were also observed. The most important biochemical changes in the serum were mild hyperproteinemia, hypoalbuminemia, hyperglobulinemia, elevated liver and kidney function, elevation in C-reactive protein and malondialdehyde level and reduction in total antioxidant capacity.
Keywords | Canine leishmaniosis; C-reactive protein; Dog protozoa; Leishmania spp.; malondialdehyde
Received | March 08, 2021; Accepted | June 02, 2021; Published | August 15, 2021
*Correspondence | Marwa M. Attia, Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: [email protected], [email protected]
Citation | Mousa SA, Attia MM, Khalphallah A, Salem NY (2021). Oxidative stress associated with canine Leishmaniosis with special reference to haemo-biochemical changes. Adv. Anim. Vet. Sci. 9(10): 1504-1510.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.10.1504.1510
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mousa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
One of the vector-borne diseases in dogs is canine leishmaniosis; a protozoan parasite transmitted mainly by sand fly (Phlebotomus papatasi) (Mauricio et al., 2018). When the cell-mediated response is deficient, propagation of the parasite occurs within macrophages of different tissues and organs (Paltrinieri et al., 2018). The disease has two main forms: cutaneous and visceral caused by members of the Leishmania genus mainly L. infantum and L. chagasi (Silva, 2007; Montargil et al., 2018). Canine leishmaniasis prevails in the old and new world (Latif et al., 2019). In Egypt, reports suggest the foci of leishmaniasis present in the Suez-canal area and Sinai Peninsula (Bessat et al.,2015). Though, the disease appears endemic in north Africa and the middle east, little information available about it in Egypt (Rosypal et al., 2013).
Variable clinical signs have been described including, cutaneous changes, enlargement of lymph nodes, glomerulonephritis, and uveitis consequent to immune-complex deposition (Maia and Campino, 2018).
Canine leishmaniosis is caused by Leishmania spp and it is considered an important zoonotic disease (Ahmadi-Hamedani et al., 2020). The severity of the disease ranged from mild self-healing skin form to more severe and may be a fatal form (Dumonteil et al., 2003). The parasite itself, the immune status, and the genes are all factors affecting the severity of the disease (Ribeiro et al., 2018). Two main systems were established for the accurate diagnosis of canine leishmaniasis, namely, LeishVet and canine leishmaniasis working group (Proverbio, 2016). The first one depends mainly on physical findings, indirect fluorescent antibody (IFT) level, and hemato-biochemical findings (Ribeiro et al., 2018). The second one depends on clinical status, cytology, PCR/histology in addition to laboratory findings (Roura et al., 2013; Paltrinieri et al., 2010).
Canine leishmaniasis (CanL) is associated with a wide range of hematobiochemical alterations, with normocytic-normochromic anemia, neutrophilia, thrombocytopenia, hypoalbuminemia, hyperglobulinemia, and elevated renal/hepatic parameters as common laboratory findings (Maia and Campino, 2018; Ribeiro et al., 2018; Ahmadi-Hamedani et al., 2020).
This study aims to identify the causative protozoan parasites infecting dogs in Egypt by discussing the clinical signs, hemato-biochemical profiles, oxidative stress, and pro-inflammatory markers alterations in CanL suspected dogs.
MATERIALS AND METHODS
Sample Collection
A total number of 100 dogs of different breeds with fever, anorexia, and lymphadenopathy were collected from January to December 2020. The age of the dogs ranged between 3-10 years. Blood samples were collected from the cephalic vein for Complete Blood Count (CBC) and serum was obtained for the determination of biochemical parameters (Zaki et al., 2021). The examined dogs were admitted to the private veterinary clinic in Giza Governorate, Egypt.
Parasitological Examination
The blood (with EDTA) was used for preparing fixed smears with methanol and stained with Giemsa stain (Attia and Mahdy, 2021). The stained blood smears were examined under the microscope (×400 and ×1000; OLYMPUS CX41) for detection of Leishmania spp (Attia et al., 2018; Zaki et al., 2021).
Hematological and Biochemical Analysis of Blood and Sera of Dogs Infected With Leishmania spp
The whole blood with EDTA underwent hematological analysis to determine different blood parameters changed during Leishmania spp infection in dogs. Serum was analyzed for the determination of total protein, albumin, ALT (Alanine amino transferase), AST (Aspratate amino transferase), AP (Alkaline phosphatase), cholesterol, triglycerides, creatinine, blood urea nitrogen (BUN), and C-reactive protein (CRP) using specific test kits (Attia et al., 2020).
Measurement of Oxidative Stress Markers
The collected sera samples from the infected dogs were subjected to analysis of malondialdehyde (MDA) and TAC (Total antioxidant capacity), using specific kits according to manufacture (Attia et al., 2020; Abdelkader et al., 2021).
Prevalence
Prevalence of affection was calculated according to the equation described by Whiting et al. (2015) as follow;
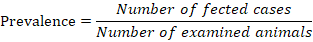
RESULTS AND DISCUSSION
Canine leishmaniasis is a vector-borne zoonotic disease with multisystem involvement (Mauricio et al., 2018). In this investigation out of examined 100 dogs with signs compatible with vector-borne diseases, the overall prevalence was 1%. Though, the disease appears endemic in north Africa and the middle east, little information available about it in Egypt (Rosypal et al., 2013).
Out of 100 dogs presented with fever, anorexia, and lymphadenopathy, only one dog was positive for Leishmania spp with an overall prevalence of 1%. The heamatological , biochemical and oxidative stress bio markers of non-infected leishmania dogs (control group = 99) were tabulated in (Table 1).
Only one case was recorded for infection with Canine leishmaniosis from 100 examined dogs (1% infection rate). The dog was a 7 years old female German Sphered dog with significant weight loss, depression, pyrexia (temp=40º), lameness on hind limbs, severe respiratory manifestations, and skin ecchymosis in the ventral abdomen, tail, and hind legs (Figure 1). Lymphadenopathy was found on physical examination. The Leishmania spp amastigotes were observed in microscopical evaluation of blood smears (Figure 2).
The dog has signs associated with different body organs. Dermatologic lesions were apparent as well as lymphadenopathy. These signs are consistent with cutaneous-visceral form. In classic cutaneous-visceral form, lymphadenopathy, followed by cutaneous lesions as non-pruritic dermatitis (with/without alopecia), erosive/ulcerative/nodular or pustular
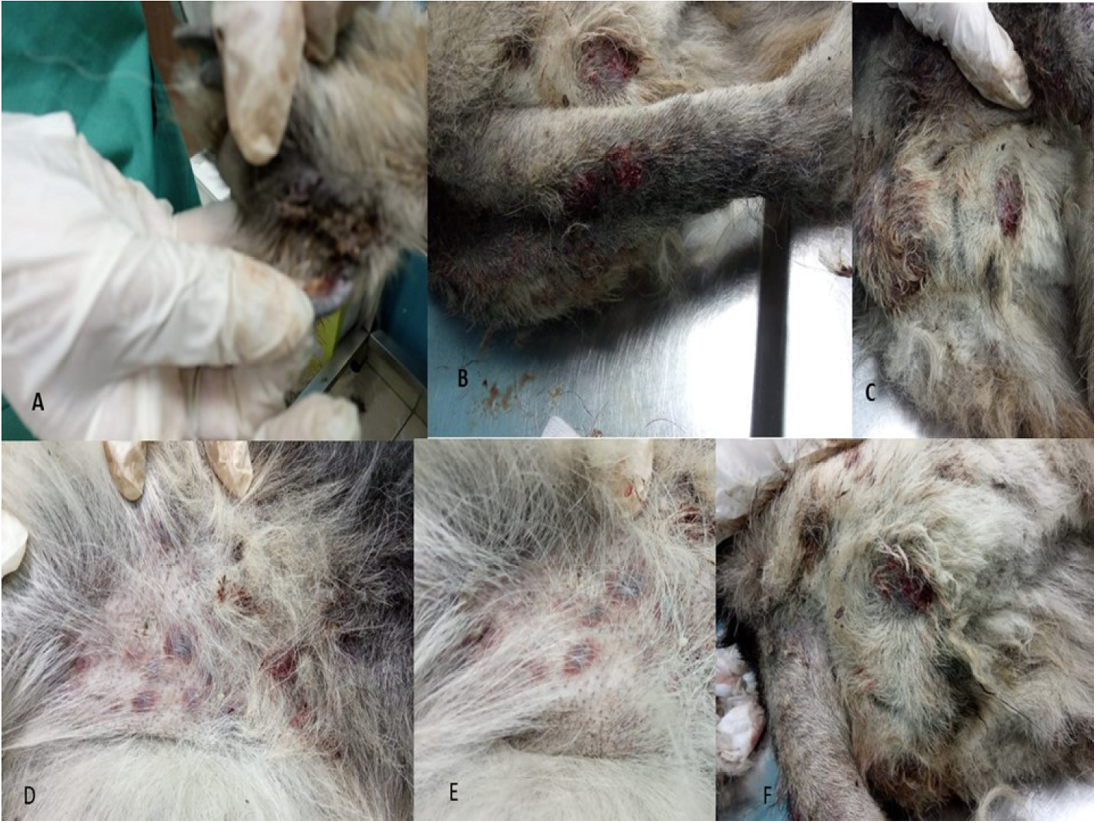
Figure 1: Clinical Signs of Dog Infected with Canine Leishmaniosis Reveals Several Skin Ecchymosis in Ventral Abdomen, Tail and Hind Legs with Lymphadenopathy.
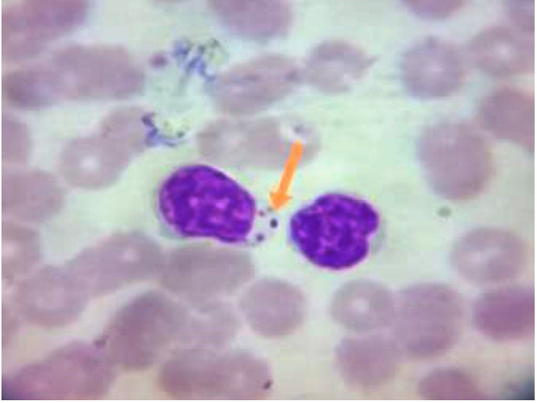
Figure 2: Blood Smear From Infected Dogs With Canine Leishmaniosis Stained with Giemsa Reveals Presence of Amastigote form of Leishmania Spp. Inside the Macrophage (Showed by Arrow).
dermatitis, and depigmentation (Lima et al., 2004; Roura et al., 2013; Koutinas et al., 2014; Ordeix et al., 2017).
Diagnosis of CanL is a complex process as the clinical signs are not present in all infected dogs (Mancianti et al., 1988). Sometimes, atypical signs such as arthritis may be present (Sbrana et al., 2014). Because of the multisystem nature of the disease, the diagnosis of the disease should not depend only on clinical and physical findings (Montargil et al., 2018; Saad and Attia, 2020).
Hematological examination revealed marked leukocytosis, neutrophilia, and monocytosis. Normocytic normochromic anemia and thrombocytopenia were also observed (Table 1).
The main alterations in hematology were a reduction in RBCs, HB, and PCV percentage, these alterations were reported in association with leishmania infections (Montargil et al., 2018). Normocytic normochromic anemia has been extensively reported (Paltrinieri et al., 2016). This type of anemia is usually seen in chronic conditions (Maia and Campino, 2018).
A reduction in erythropoiesis by bone marrow or consequent to renal disease with an expected reduction in erythropoietin hormone, or hemolysis may be implicated (Maia and Campino, 2018). Though some reports showed normocytic hypochromic anemia (Torrecilha et al., 2016), and iron deficiency could be implicated (Jain, 1986).
Table 1: Hematological Parameters in LEISHMANIA Non -Infected Dogs ( Control Group) and Leishmania-Infected dog Compared to Normal Reference Range.
| Parameter | Non-infected dog (control group=99) | Diseased dog | Reference range* (SI units) | ||
| M ± SE | Minimum Value | Maximum Value | |||
|
RBCs (×1012/L) |
7±0.09 | 5.40 | 8.50 | 4.09 | 5.5- 8.5 |
| PCV | 0.46± 0.60 | 0.38 | 0.58 | 0.31 | 0.37-0.55 |
| HB (g/l) | 155.6± 0.15 | 129 | 186 | 104 | 120-180 |
| MCV ( fl) | 66.79± 1.19 | 49.24 | 99.31 | 75.7 | 60-77 |
| MCHC (g/l) | 340.2± 0.31 | 263.8 | 457.7 | 335.4 | 320-360 |
|
Thrombocytes (103/μL) |
199.01± 2.72 | 160.30 | 250 | 122 | 160-430 |
|
WBCS (103/μL) |
9.48± 0.14 | 7.20 | 12.30 | 17.590 | 6-17 |
|
Neutrophils (103/μL) |
8.68± 0.11 | 3 | 10.30 | 12.840 | 3-11.5 |
|
Lymphocytes (103/μL) |
3.34± 0.05 | 2.20 | 4.20 | 2.638 | 1-4.8 |
|
Monocytes (103/μL) |
1.42± 0.005 | 1.31 | 1.51 | 1.759 | 1.350-1.50 |
|
Eosinophils (103/μL) |
1.44± 0.04 | 1.31 | 1.51 | 3.518 | 1.350-1.50 |
*Reference values were extracted from ‘Jain, 1986; Kaneko et al., 1997; Todorova et al., 2005; Willard and Tvedton, 2012 and Almeida et al., 2013.
Leukocytosis, neutrophilia, and monocytosis were detected in the affected dog. The response of the body to various organ assaults and inflammation by the parasite is neutrophilia (Torrecilha et al., 2016). ‘Zandbergen et al. (2004) described the neutrophil in Leishmania infection as “trojan-horse”. The elevation in both leucocytes and neutrophils could be attributed to secondary bacterial infection or the over-activation of the cellular immune response at this point overrules leucocyte destruction (Montargil et al., 2018). Thrombocytopenia was another finding in hematological alterations, and it was described in association with Leishmania infection (Paltrinieri et al., 2016). The reported hematologic parameters varied in previous reports, for instance, Paltrinieri et al. (2010) reported Macrocytic-hypochromic anemia, monocytosis, leukopenia, neutrophilia, proteinuria, and thrombocytopathy; the same type of anemia was also reported by Melendez-Laso et al. (2018). However, Ahmadi-Hamedani et al. (2020) reported Anemia, neutrophilia, monocytosis, and leukocytosis. Moreover, Torrecilha et al. (2016) reported Non-regenerative anemia, eosinopenia, and neutrophilia.
Table 2: Serum Biochemistry, Oxidative Stress, and Inflammatory Biomarkers In Leishmania Non -Infected Dog (Control Group) and Leishmania-Infected Dog Compared to Normal Reference Range.
| Parameter | Non-infected dog (control group= 99) | Diseased dog | Reference range* (SI units) | ||
| M± SE | Minimum Value | Maximum Value | |||
| TP(g/L) | 63.2± 0.07 | 51 | 75 | 72.8 | 54-71 |
| Albumin (g/l) | 28.80± 0.02 | 24 | 33 | 20.8 | 26-33 |
| Globulin(g/l) | 34.4± 0.07 | 18 | 51 | 52 | 27-44 |
| A/G ratio | 0.89± 0.02 | 0.47 | 1.83 | 0.4 | |
| ALT (µkat/L) | 0.90± 2.08 | 0.33 | 1.54 | 1.62 | 0.17-1.57 |
| AST (µkat/L) | 0.88± 0.77 | 0.67 | 1.10 | 1.41 | 0.38-1.10 |
| ALP (µkat/L) | 2.27± 0.95 | 2 | 2.54 | 3.40 | 0.33-2.61 |
| Cholesterol (mmol/L) | 5.94± 1.20 | 5.44 | 6.50 | 5.53 | 3.50-6.99 |
| Triglycerides(mmol/L) | 1.12 ± 0.58 | 1.02 | 1.24 | 1.11 | 0.23-127 |
| BUN (mmol/L) | 15.84± 0.91 | 10.71 | 21.42 | 21.78 | 3.57-17.85 |
| Creatinine (µmol/L) | 58.34± 0.01 | 44.20 | 78.68 | 147.63 | 44.20-123.76 |
| CRP (mg/l) | 30.5± 0.12 | 10 | 60 | 180 | ≤ 60 |
| TAC (m mole/L) | 0.95± 0.003 | 0.90 | 1 | 0.608 | 0.9-1 |
| MDA (m mole/L) | 1.13± 0.05 | 0.60 | 2.30 | 2.872 | 0.659-2.5 |
*Reference values were extracted from ‘Jain, 1986; Kaneko et al., 1997; Todorova et al., 2005; Willard and Tvedton, 2012 and Almeida et al., 2013.
The present study reported elevation of serum activities of ALT, AST, and ALP in dogs with leishmaniasis compared with the reference value. The serum concentration of blood urea nitrogen was slightly increased, also there was a slight elevation in MDA levels (Table 2).
A slight elevation in total protein along with a reduction in albumin and elevated globulin were recorded. Elevated liver and kidney markers were also observed. Alterations in liver enzymes, azotemia, and disruption in protein balance, with elevation in protein caused by elevated globulin, caused mainly by the response of polyclonal B cell and reduction of albumin caused by hepatocyte damage and renal disease (Heidarpour et al., 2012; Salem et al., 2014; Ribeiro et al., 2013; Paltrinieri et al., 2016). Elevation in renal parameters was observed. In another protozoan model (Babesia), it was believed that renal degeneration with subsequent necrosis could play a role in elevated renal parameters (Mosqueda et al., 2012). A non-kidney-related elevation could be also implicated as a result of hemorrhage and hemolysis (Reyes et al., 1998). CanL is associated with glomerulonephritis, a disease of the kidney associated with alterations in renal parameters (Maia and Campino, 2018). Increased levels of liver enzymes are consistent with liver injury (Graham et al., 2003; Ramadan et al., 2019).
Oxidative Stress Parameters
Elevated MDA and reduction in TAC were recorded. Lipid peroxidation products as MDA elicits an oxidative stress process which in turn, exacerbates the inflammation (Abdelnaby et al., 2020). As the disease progress, apoptosis of cells increases, and antioxidant components of neutrophils will be reduced (Almeida et al., 2013a). Torrecilha et al. (2016) observed a positive correlation between parasitic load and TAC. TAC is used as an easy and inexpensive way to give a crude idea about antioxidant status in the body (Abdel-Saeed and Salem, 2019). Leishmania causes oxidative stress (Maia and Campino, 2018), at the site of inflammation, phagocytes will release reactive oxygen spp. (ROS), leading to activation of antioxidant components, which will be consumed in fighting formed ROS (Torrecilha et al., 2016; Kubesy et al., 2017; Elsayed et al., 2020). This mechanism is hypothesized as a way for the parasite to elude the body’s immune system (Torrecilha et al., 2016). ‘Heidarpour et al. (2012) observed an elevation in the oxidant side and reduction in the antioxidant side in dogs with clinical signs.
Pro-Inflammatory Markers
Changes in an acute phase proteins (APPs) were observed with elevated positive APP (CRP) and reduction in negative APP (albumin); these alterations were recorded in Martinez-Subiela et al. (2014), Paltrinieri et al. (2016). Hypoalbuminemia is considered a negative prognostic marker (Paltrinieri et al., 2016).
CONCLUSION
Cuteno-visceral form of Canine leishmaniasis (CanL) is associated with marked hemato-biochemical alterations, oxidative stress, and disruption of pro-inflammatory markers. No specific treatment to CanL but symptomatic treatment is recommended with control of the vector of the protozoan parasites.
AUTHORS CONTRIBUTION
All authors have made substantial contribution in conception and design of the study. They have been involved in drafting, revising and final approval of the manuscript. Marwa M. Attia identified and photographed the parasites.
Conflict of Interest
The authors declare that they have no conflict of interest. No funding was supporting this work.
REFERENCES






