Advances in Animal and Veterinary Sciences
Research Article
Relationship Between the Appearance of First Estrus (Puberty) with Leptin and Body Conditions Score (Bcs) Levels in Bali Cattle
Luh Gde Sri Surya Heryani1*, Desak Nyoman Dewi Indira Laksmi2, Devi Latifah Puji Lestari3, I Gusti Ngurah Bagus Tri Laksana2, Luh Made Sudimartini4, I Wayan Nico Fajar Gunawan4
1Department of Veterinary Anatomy; 2Departement of Veterinary Reproduction; 3Student of Faculty of Veterinary Medicine; 4Department of Veterinary Clinic, Faculty of Veterinary Medicine Udayana University Jl. PB. Sudirman, Denpasar, Bali 80223, Indonesia.
Abstract | The appearance of puberty needs the GnRH secretion, due to the high amplitude and frequency of the GnRH pulsatile and the estradiol feedback system of the ovary with the hypothalamus. However, there are things to note, which is the complex system of neural pathways, neurohormones, and peptides that modulate the secretion of GnRH itself and mediate the effect of estradiol on GnRH. In line with the increasing age and growth of heifers, some factors induce and interact with various metabolic signals, one of which is leptin, where its receptors know leptin in the central nervous system. Leptin concentration increases during puberty development. However, leptin concentration must reach a certain threshold to activate the axis of the ovarian pituitary hypothalamus. This study aims to determine leptin levels, and body conditions score (BCS) when the first estrus emergence (puberty) and find out the first estrus quality (puberty) in bali cattle. This study is an analytic observational study with cross-sectional Study design. The sample used is cattle at puberty. The research sample had a health status that showed no signs of illness. The collection of blood samples from Balinese cattle was carried out in several simantri in Mengwi District, Badung Regency. Parameters measured were leptin levels and physical signs of estrus, namely the presence of transparent coloured springs. Techniques for measuring hormone levels with the Direct Elisa method, Double Antibody Sandwich. The result showed that the level of leptin when the appearance of the first estrus (puberty) in Bali cattle is an average of 5.62 ng/ml, the average of body condition score (3.06), and puberty age (21.44 months). Correlation test showed a correlation between body condition score and the appearance of the first estrus with regression correlation value (r) 0.852. Correlation test showed a correlation between the levels of leptin hormone and the appearance of the first estrus with the regression correlation value (r) 0.805. If r approaches 1 or -1, the relationship between the two variables is strong, and there is a high correlation.
Keywords | Puberty, Leptin, Bali cattle, GnRH, Body conditions score
Received | June 25, 2019; Accepted | July 26, 2019; Published | September 25, 2019
*Correspondence | Luh Gde Sri Surya Heryani, Department of Veterinary Anatomy, Faculty of Veterinary Medicine Udayana University, Bali, Indonesia; Email: [email protected]
Citation | Heryani LGSS, Laksmi DNDI, Lestari DLP, Laksana IGNBT, Sudimartini LM, Gunawan IWNF (2019). Relationship between the appearance of first estrus (puberty) with leptin and body conditions score (bcs) levels in bali cattle. Adv. Anim. Vet. Sci. 7(10): 904-909.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.10.904.909
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Heryani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
One of the reproductive efficiency parameters is the achievement of the early age of puberty that similar to its genetic potential. This is important for achieving optimum cow reproduction performance and increasing productivity. Information on the age of early puberty is also important as a reference in improving reproductive efficiency based on potential and problems in the field through various technological innovations.
Puberty is controlled by specific physiological mechanisms involving the gonads and adenohypophysis glands, so that puberty does not escape the influence of hereditary factors and the environment that works through these organs (Toelihere, 1995), environment (nutrition, climate and season) and males or biostimulation (Rekwort et al., 2000; Getzewick 2005; Abdelgadir et al., 2010). Body weight is considered to play an important role in regulating the appearance of puberty (Maciel et al., 2003). The body weight is related to nutrition in the cow. One way to see the level of nutrition in cattle is to look at the body condition score (BCS).
Adipose tissue mass and weight are considered to play an essential role in regulating puberty onset (Maciel et al., 2003). In prepubertal ruminants, short-term dietary restrictions will reduce the expression of the leptin adipose gene and leptin secretion. (Amstalden et al., 2002). In this case, the limitations of nutrition will inhibit LH secretion. However, giving leptin will restore LH secretion. The effect shows a positive relationship between LH and leptin secretion (Amstalden et al., 2002). Furthermore, leptin concentrations increase as well as leptin gene expression in cows during the development of puberty along with increased IGF-I level and weight (Garcia et al., 2002).
The administration of leptin has been studied to stimulate puberty in rodent animals. Leptin injection can stimulate puberty earlier in mice by increasing the reproductive tract maturation (Chehab et al., 1997; Cheung et al., 1998). However, the hormones and metabolism that connects nutrition and puberty, as well as the neuroendocrine mechanism by which GnRH neurons are stimulated to increase secretory activity resulting in the first puberty ovulation, have not been explained in cattle.
The administration of leptin has been studied to stimulate puberty in rodent animals. Leptin injection can stimulate puberty earlier in mice by increasing the reproductive tract maturation (Chehab et al., 1997; Cheung et al., 1998). However, the hormones and metabolism that connects nutrition and puberty, as well as the neuroendocrine mechanism by which GnRH neurons are stimulated to increase secretory activity resulting in the first puberty ovulation, have not been explained in cattle.
MATERIALS AND METHODS
This study is an analytical observational study with a Cross-Sectional Study design. The samples used were 18 heifers. The heifers were fed with fresh elephant grass (Pennisetum purpeum) (20kg/animal/day) with 10% (2kg/animal/day) additional pellet (CalFeed P-122+®) and the drink was given ad libitum. The sample is healthy and showed no signs of disease. Blood samples were taken through the jugular vein. The blood was inserted in a tube without anticoagulant and then left for 15 minutes, then centrifuge at 3,000-4,000 rpm for 15-20 minutes, to obtain serum. The obtained serum was stored at -20 C until the examination of leptin levels was carried out. Estrus observation is done twice a day, every morning (06:00-09:00 AM) and afternoon (04:00-06:00PM) with estrus signs that indicate anxiety, the vulva swells, and redness also covered by transparent mucus, increase of blood to the vaginal mucosa and accumulation of mucous-containing fluid in the vagina, urination, and ready to accept males (Hafez and Hafez, 2000).
Data were analyzed using the SPSS version 20 for Window, including; normality test with Kolmogorov-Smirnov, homogeneity test with Leven’s Test, and Correlation and Regression test to find out the relationship and uniformity between leptin levels and the appearance of the first estrus (puberty).
RESULT AND DISCUSSION
The average levels of leptin in Bali cattle raised in several simantri (farmer groups) in Sobangan village, Badung Regency showed that the appearance of puberty in Bali cattle were ranged from 20.80 months with an average leptin level of 5.62 ng/ml (Table 1).
Table 1: Average (x ± SD) of Leptin and BCS Level (ng /ml) and Age (month) The Appearance of Puberty in Bali Cattle.
| Age | BCS | Leptin Level |
| 20.80 ± 2.43 | 3.06 ± 0.725 | 5.62 ± 0.24 |
Relationship Between Age of Puberty and Levels of Leptin Hormones
Table 2: Relationship between levels of leptin hormone and puberty in bali cow.
| R | R Square | Adjusted R Square | Std. Deviation of the Estimate |
| 0.826 | 0.682 | 0.627 | 10.307 |
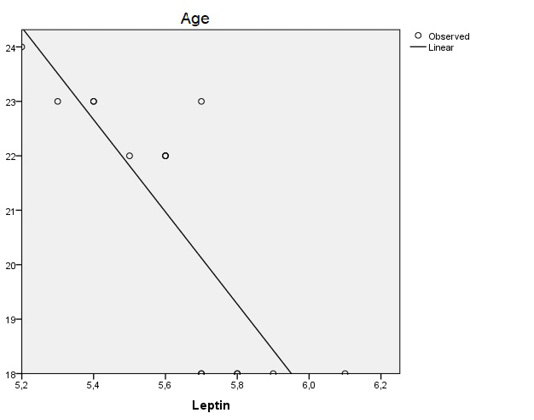
Statistical analysis of the relationship between the age of puberty and levels of leptin hormone showed on table 2. The results of the correlation coefficient (r) = 0.826 and the coefficient of determination (r2) = 0.682 with the regression line equation y = 68.39 + 8.46 x, where y is age and x is leptin (figure 1).
The graph shows that the age of puberty is closely related to the levels of leptin and contributes 68.2% to the levels of leptin. Increasing age by 1 point causes an increase in leptin levels of 8.46%.
The appearance of puberty is related to achieving optimal weight or a minimum percentage of body fat. In addition, the correlation between metabolic and food intake triggers the mechanism of emergence of puberty. Metabolic signals are important in puberty initiation. Leptin functions as a metabolic signal of nutritional status that activates the axis of the ovarian pituitary hypothalamus. Leptin is a peptide produced by adipose tissue, which is the primary connection between nutrition, metabolism, and reproduction (Garcia et al., 2002).
Serum leptin concentrations increase as well as leptin gene expression in cattle during the development of puberty together with an increase in serum IGF-I level and weight (Garcia et al., 2002). However, there are differences in adult cattle that are not fed in the short term, failing to reduce pulsatile LH secretion (Amstalden et al., 2002). This shows that there is an increased sensitivity from the hypothalamic-pituitary axis to variations in energy availability in the heifers.
Leptin serum increases at puberty in rodents and humans (Ahima et al., 1997; Quinton et al., 1999). Increased body weight, seasonal changes, and serum leptin-binding protein are variables that have been shown to contribute to the increased circulation of leptin (Maffei et al., 1995; Bocquier et al., 1998; Housknecht et al., 1996).
In conditions of malnutrition, inhibition of LH secretion can be overcome by administering leptin. The effect shows a positive relationship between LH and leptin secretion (Amstalden et al., 2002). Serum leptin concentrations increase during puberty in rats, pigs, and cattle (Garcia et al., 2002). In prepubertal ruminants, limited feed reduces leptin gene expression in adipose tissue and leptin secretion, but increases hypothalamic OB-Rb expression. This is related to a decrease in serum insulin, IGF-I, and LH pulse frequency (Amstalden et al., 2002). Although serum leptin concentrations increase during puberty in the female pigs, there are other factors besides leptin that can regulate the appearance of puberty. Puberty is related to GnRH, LH, ovary E2, and hypothalamic sensitivity to E2, and neurohormones. Estradiol modulates the hypothalamic-pituitary response to leptin. Estradiol regulates changes in puberty related to leptin gene expression (Ahmadzadeh et al., 2013).
Many factors, directly or indirectly affect puberty. These factors include genetics and breed, weight, and level of weight gain, body composition, nutrition and feed, and certain environmental or social conditions such as seasons, photoperiods, and the presence or absence of bulls. Feed shortages in animals will cause delays in puberty, while excess feed will shorten puberty. An adequate feed is needed for normal endocrinal function. The synthesis and secretion of reproductive hormones by endocrine glands are influenced by the level of feed given, the more quality and the sufficient amount of feed given, the faster the synthesis of hormones. Growth and development of reproductive organs will be delayed if young female animals experience a lack of feed, both quality, and quantity. (Ahmadzadeh et al., 2013).
In Brahman heifers that were feed with two moderate nutrition (MN) and improved nutrition (IN). All 11 heifers on MN attained puberty between 16 and 23 months of age and only one of 11 heifers on MN had ovulated by 23 months of age. Heifers on IN achieved puberty at 22.0 ± 0.8 months of age, 398 ± 13 kg BW and BCS of 4.04 ± 0.14. (Samadi et al., 2014).
However, administration of recombinant leptin for 40 days did not stimulate an increase in LH pulse frequency in prepubertal cattle with moderate to heavyweight. The lack of influence of leptin on LH pulse frequency and its failure to accelerate the emergence of puberty. It suggests that administration of leptin is not effective in increasing the pulse frequency of GnRH or chronically increases LH through its direct effect on the pituitary (Amstalden et al., 2002, 2003). Therefore, the acute and chronic limited energy for hypersensitization of the hypothalamic-hypophysis axis into leptin. Therefore, administration of leptin does not affect the pattern of LH secretion in cattle that is adequately fed, which will inhibit neuro-endocrine triggers for puberty (Maciel., 2013).
Thus, leptin concentration increases in circulation during the development of puberty and reaches a level that allows for activation of the ovarian pituitary-hypothalamic axis. Leptin does not function as a trigger signal but acts primarily as a permissive signal that allows puberty to occur. Therefore, in ruminants, leptin 1) acts primarily as a passive hormone that allows puberty to occur when sexual maturity is reached; and 2) functions as metabolic signals that can regulate gonadotropin secretion in response to limited energy both acute and chronic (Maciel et al., 2013).
Relationship Between Age of Puberty and Levels of BCS
Table 3: Relationship Between Age of Puberty and Levels of body condition score (BCS) in Bali Cattle
| R | R Square | Adjusted R Square | Std. Deviation of the Estimate |
| 0.852 | 0.725 | 0.708 | 1.052 |
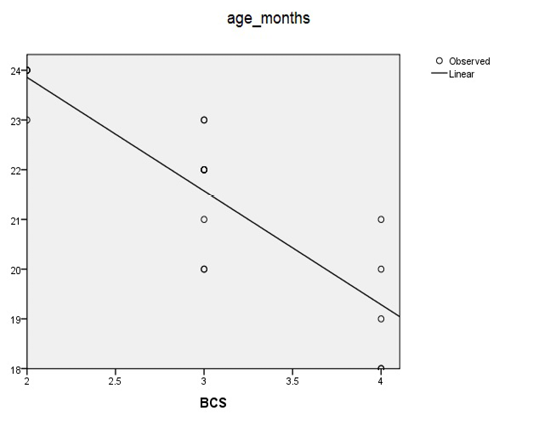
Table 3 and Figure 2 show that there is a relationship between body condition score (BCS) with the age of puberty of bali cow maintained at several simantri in Mengwi Subdistrict, Badung Regency (P <0.01). The results of the correlation coefficient (r) body condition score (BCS) with the age of bali cow puberty were 0.852. If r approaches 1 or -1, the relationship between the two variables is secure, and there is a high correlation. Whereas if r is close to zero, then linear relations X and Y are weak, or maybe none at all (Imamul et al., 2007). X is the body condition score, and Y is the age of puberty in bali cow.
In this study, there was a relationship between body condition score (BCS) and leptin hormone levels on puberty age in bali cow maintained in several simantri in Mengwi District, Badung Regency (P <0.01). The results of the correlation coefficient (r) body condition score (BCS) with the age of bali cow puberty were 0.852. The result of the correlation coefficient (r) of leptin hormone with age of puberty in bali cow is 0.805. If r approaches 1 or -1, the relationship between the two variables is strong, and there is a high correlation. Whereas if r is close to zero, then linear relations X and Y are weak, or maybe none at all (Imamul et al., 2007).
The appearance of puberty is related to achieving optimal weight or a minimum percentage of body fat. In addition, the correlation between metabolic and food intake triggers the mechanism of emergence of puberty. The important metabolic signal in puberty initiation. Leptin functions as a metabolic signal of nutritional status which activates the axis of the ovarian pituitary hypothalamus. Leptin is a peptide produced by adipose tissue, which is the main link between nutrition, metabolism, and reproduction (Garcia et al., 2002). Laksmi (2016), states that there is a close relationship between exogenous leptin hormone and endogenous leptin hormone (r = 0.831). The administration of exogenous leptin hormone can increase endogenous leptin levels, which will increase the development of ovarian follicles and accelerate the emergence of postpartum estrus.
Many factors, directly or indirectly affect puberty. These factors include: genetics and breed, weight, and level of weight gain, body composition, nutrition and feed, and certain environmental or social conditions such as seasons, photoperiods, and the presence or absence of bulls (Rekwort et al., 2000; Getzewick, 2005; Abdelgadir et al., 2010). In this research the heifers were given with fresh elephant grass in Bali which has protein value 10.32% and fiber 22,65% (Sudita, 2016) and with the addition of pellet which has protein value 14%. The higher nutrition from the feed especially in protein value could short the puberty in heifers. In contrast, feed shortages in animals will cause delays in puberty, while excess feed will shorten puberty (Stone et al., 1996). An adequate feed is needed for normal endocrinal function. The synthesis and secretion of reproductive hormones by endocrine glands are influenced by the level of feed given, the more quality and the sufficient amount of feed given, the faster the synthesis of hormones. Growth and development of reproductive organs will be hampered if young female animals experience a lack of feed both in quality and quantity (Ahmadzadeh et al., 2013).
In normal mice, humans, sheep, and cattle, leptin concentrations are positively correlated with adiposity. In prepubertal cows, leptin concentrations correlate with weight gain (Amstalden, 2003). Leptin in the blood is strongly correlated with the accumulation of the amount of adipose tissue. Reduced nutrition will reduce the level of adipose mRNA leptin tissue in cattle (Agarwal et al., 2009). In ruminants, circulating leptin concentrations are positively correlated with obesity, but this relationship only explains about 10-30% of the variation in leptin concentration. This means that there are other factors also play an important role (Knop and Cernescu, 2009).
Concerning body condition score (BCS) this study is in line with Lents et al. (2005), that the condition of the body condition of a cow significantly influences the concentration of the leptin hormone. However, this is not in line with the statement of Mappanganro et al. (2014) which states that there is no close relationship (P> 0.05) between the leptin gene and the body condition scores of bali cows and their crosses.
Body condition score is a method of subjective assessment through visual and touching techniques to predict food reserves (Lents et al., 2005). Gentry et al. (2002) state that livestock that has very thin or obese bodies can be caused by lack of nutrition, excess nutrition, health problems, or improper management. The body condition score (BCS) can be used as a benchmark that is related to the nutrition of bali cow.
Adipose tissue mass and weight are considered to play important roles in regulating the appearance of puberty (Maciel et al., 2003). According to Laksmi (2018), the body condition score (BCS) has a relationship with the levels of leptin hormone in Bali cattle experiencing postpartum anesthesia. Also, Vargova et al. (2016) reported that there was a relationship between plasma leptin and body condition score (BCS) in the cow during lactation.
CONCLUSION
The level of leptin when the appearance of the first estrus (puberty) in Bali cattle is an average of 5.62 ng/ml. There is a close relationship between the age of emergence of puberty and leptin levels in bali cattle with a correlation level (r) = 0.826, and between age and BCS with the correlation coefficient (r) of body condition score (BCS) with the age of bali cattle puberty was 0.852. The correlation between BCS and age provides an insight about the beginning of puberty achieved the BCS is getting better. This can be achieved by providing a high nutritional food intake.
ACKNOWLEDGEMENT
The author would like to thank the Ministry of Research, Technology, and Higher Education, through Udayana University for the research funds provided so that this research can be completed as expected.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Authors contribution
Luh Gde Surya Heryani designed the study, analyzed and drafted the manuscript. Devi Latifah Puji Lestari and Luh Made Sudimartini collected the data. Desak Nyoman Dewi Indira Laksmi and I Gusti Ngurah Bagus Tri Laksana interpreted the data. I Wayan Nico Fajar Gunawan took part in preparing and critical checking of the manuscript.
REFERENCES