Research Journal for Veterinary Practitioners
Research Article
Detection of Intestinal Protozoa in Camels and their Breeders in Najef, Iraq
Asmaa Ghafer Hussin1, Jenan Mahmood Khalaf1, Haider Mohammed Ali2
1Department of Zoonotic Unit; 2Department of Parasitology, College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | The study aimed to investigate the existence of intestinal protozoa in feces of the camels and their breeders in Najef province, Iraq because this province is having a large number of camels. One hundred fecal samples were collected from camels (33 males and 67 females) aged between >3 to 6≤ year and 25 fecal samples were collected from camels breeders (14 males and 11 females) aged between >30 to 60 years during November 2014 to May 2015. Samples were examined through direct wet smear and Lugal’s iodine for cycts or trophzoite of Giardia spp. and Entameba spp. while modified Ziehl–Neelsen staining technique for oocycts of Cryptosporidium spp. The results showed that the infection rates of Cryptosporidium spp., Giardia spp. and Entameba spp. in camels were 61%, 24%, and 20%, respectively. The corresponding infection rates in camel-breeders were 56%, 20%, and (16%), respectively. It can be concluded that the high prevalence values of the intestinal protozoa were detected in camels and camel-breeders. The prevalence values seems to be parallel between camels and camel-breeders who are mainly in touch with camels. This could be an evidence of the association of infection between camels and camel-breeders.
Keywords | Intestinal protozoa, Camels, Camel-breeders, Najef
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | June 14, 2015; Revised | July 02, 2015; Accepted | July 03, 2015; Published | July 06, 2015
*Correspondence | Asmaa Ghafer Hussin, University of Baghdad, Iraq; Email: [email protected]
Citation | Hussin AG, Khalaf JM, Ali HM (2015). Detection of intestinal protozoa in camels and their breeders in Najef, Iraq. Res. J. Vet. Pract. 3(3): 53-57.
DOI | http://dx.doi.org/10.14737/journal.rjvp/2015/3.3.53.57
ISSN | 2308-2798
Copyright © 2015 Hussin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Camels (Camelus dromedaries) are generally infected with numerous parasites. Parasitic infection cause considerable losses in camels as a result of mortality. Several types of protozoa are responsible for enteric infections in camels (Wahba and Refail, 2003). Enteric protozoan parasites are ubiquitous in domestic mammal populations (Taylor, 2000). Many species have intracellular life-cycle phases in the intestinal epithelia and have the potential to cause disease. Their widespread occurrence, economic importance coupled with limited options for treatment and sometimes zoonotic potential have meant most investigations of protozoan infections in ruminants have come from outbreaks on farms or from experiments involving production animals (Craig et al., 2007). Intestinal protozoa are increasingly being studied because of their association with acute and chronic diarrhoea in immunocompromised as well as immunocompetent patients (Mak, 2004). Various community outbreaks due to contamination of water or food with these protozoa have further highlighted their importance in public health (Mak, 2004). Intestinal parasites, caused by protozoa represents one of the major causes of impaired milk production, as well as impaired fertility and low calving rates of camels (AL-Megrin, 2015). The aim of this study is to investigate the effect of some factors on infection rate of Cryptosporidium spp., Giardia spp. and Entameba spp. in camels and camel’s breeders along with to identify the association infection between them.
MATERIALS AND METHODS
Stool Collection
Samples of 5-10 grams were collected randomly from different ages and sex of camels besides samples of camel’s breeders in the Najef province/Iraq. All these samples collected from November, 2014 to May, 2015. The fecal samples were collected directly from the rectum, in a clean plastic containers (100ml size) and were tightly closed and given sequential numbers. All information for the animal included age and sex were recorded on the special form cart. The samples were transported in refrigerated bag to the laboratory (Zoonotic Unit at the College of Veterinary Medicine, University of Baghdad).
Direct Wet Mount And Lugol’s Iodine Staining Methods
A portion of stool was examined at field by direct transport to the wet mount with saline (0.85% sodium chloride solution) to observe motile intestinal parasites (Cryptosporidium spp., Giardia spp. and Entameba spp.) and trophozoites under light microscope at 10X and 40X magnifications. Lugol’s iodine staining technique was also done to observe cysts of the intestinal protozoan parasites (Levine, 1961).
Modifieed Zeihl-Neelsen Method
A smear was also made from the fresh stool samples on glass slide and stained with Modified Ziehl-Neelsen acid-fast stain as described by (Garcia, 2001) for the identification of the oocysts of Cryptosporidium parvum using the x40 and x100 objectives.
Statistical Analysis
Statistical analysis was performed by SPSS software. Chi-square test was used to detect the association between each of age and sex with infection rate of Cryptosporidium, Giardia and Entameba spp. P value less than 0.05 were considered statistically significant.
RESULTS
Cryptosporidium spp., Giardia spp. and Entameba spp. Infection
In Camels According to Sex
The results revealed that out of (100) fecal samples collected from camels in Najef province were as follows; total positive cryptosporidiosis percentage in the males and females were 33(63.63%) and 67(59.70), respectively. The prevalence of giardiasis was 18.18% and 26.86% in the males and females respectively. The corresponding prevalence of entamebaiasis was 18.18% and 20.89% respectively. The highest prevalence was detected in Cryptosporidium spp. (61%), whereas the lowest was found in Entameba spp. (20%) and Giardia spp. (24%). On the other hand the differences between males and females were not significant across the three protozoa (Table 1).
The prevalence of Cryptosporidium spp. in camels (61%) in the present study was higher than prevalence reported by several researchers for the same animal; 37.9% in Iran (Razavi et al., 2009), 17.5% and 19.3% (El Kelesh et al., 2009; Abdel-Wahab and Abdel–Maogood, 2011) in Egypt.
The prevalence of Giardia in camels was 24% which was too lower than 100% documented in Iraqi by Radhy et al. (2013). On the other hand Beck et al. (2011) confirmed the absence of Giardia in Croatia. These differences could be attributed to difference in environment condition between the countries besides the difference in the number of camels included in these studies. Similarly, the prevalence of Entameba spp. (20%) was not much differed from (17.85%) reported by Rewatka et al. (2009) in migratory.
In Camels according to Age
In Table 2, it was shown that highest estimations of Cryptosporidium spp. and Entameba spp. were 75% and 29.16% in age (3-<6 years), respectively. While the highest estimation of Giardia spp. was 29.41% in age 6≤ years. Statistical analysis shows that the differences in prevalence among age categories are significant (P < 0.01) in Cryptosporidium spp. and Entameba spp. (P < 0.05), while the differences in prevalence of Giardia spp. was not significant.
The highest infection rate of Cryptosporidium spp. in camels found at age (3≤ 6 years). These results agreed with the previous studies which showed that the highest rate of infection appeared at age group of (2-<4) years (Hamza, 2007) in AL-Qadysiya Province, Iraq. Also this percentage was almost compatible with the result obtained by Razavi et al. (2009) in Iran who found that the highest percentage was at the age of <5 years. The high prevalence rate of Cryptosporidium spp. in camels in our study could be attributed to the risk of infection through environmental contamination due to grazing with other infected animals or to the spreading of manure (Razavi et al., 2009).
The highest infection rate of Giardia spp. was found at the 6≤ age. This could be due to increase susceptibility to the Giardia infection with age progress because of increased exposure to causative agent with increased water and feeds intake, also the change in the physiological status in females such as parturient and suckling periods as well as, the difference of hormonal excretion, all these represent a stress factors which caused a decline in immunity level and then would increase to the opportunity of infections. The highest infection rate of Entameba spp. (29.16% ) at age 3- 6< year was higher than the 17.85% recorded by Rewatka et al. (2009).
Among Camel’s Breeders according to Sex
Table 3 showed the prevalence of Cryptosporidium spp., Giardia spp. and Entameba spp. of camel’s breeders according to sex. The infection rates of Cryptosporidiosis in males and females were 57.14% and 54.54%, respectively. The prevalence of giardiasis was 21.42% in the males and 14.28% in the females. While the prevalence of Entamebaiasis was 14.28% and 18.18% in the males and females respectively. The highest prevalence was found in Cryptosporidium spp. (56%), whereas the lowest was found in Entameba spp.
Table 1: Prevalence of Cryptosporidium spp., Giardia spp. and Entameba spp. in camels according to sex
|
Sex |
Total animals |
Cryptosporidium spp. |
Giardia spp. |
Entameba spp. |
|||
|
No. of Positive |
(%) |
No. of positive |
(%) |
No. of positive |
(%) |
||
|
Males |
33 |
21 |
63.63 |
6 |
18.18 |
6 |
18.18 |
|
Females |
67 |
40 |
59.70 |
18 |
26.86 |
14 |
20.89 |
|
Total |
100 |
61 |
61 |
24 |
24 |
20 |
20 |
|
Chi sq |
0.14 |
0.91 |
0.10 |
||||
|
P value |
0.70 |
0.33 |
0.75 |
||||
Table 2: Prevalence of Cryptosporidium spp., Giardia spp. and Entameba spp. in camels according to age
|
Age/Year |
Total animals |
Cryptosporidium spp. |
Giardia spp. |
Entameba spp. |
|||
|
No. of Positive |
(%) |
No. of positive |
(%) |
No. of positive |
(%) |
||
|
<3 |
42 |
28 |
66.66 |
10 |
23.80 |
11 |
26.19 |
|
3-<6 |
24 |
18 |
75 |
4 |
16.66 |
7 |
29.16 |
|
6≤ |
34 |
10 |
29.41 |
10 |
29.41 |
2 |
5.88 |
|
Chi sq |
15.21 |
1.25 |
6.50 |
||||
|
P value |
<0.01 |
0.53 |
<0.05 |
||||
Table 3: Prevalence of Cryptosporidium spp., Giardia spp. and Entameba spp. infection among breeders contact with camels according to sex of breeders
|
Sex |
Total breeders |
Cryptosporidium spp. |
Giardia spp. |
Entameba spp. |
|||
|
No. of Positive |
(%) |
No. of positive |
(%) |
No. of positive |
(%) |
||
|
Males |
14 |
8 |
57.14 |
3 |
21.42 |
2 |
14.28 |
|
Females |
11 |
6 |
54.54 |
2 |
18.18 |
2 |
18.18 |
|
Total |
25 |
14 |
56 |
5 |
20 |
4 |
16 |
|
Chi sq |
0.01 |
0.04 |
0.06 |
||||
|
P value |
0.89 |
0.84 |
0.79 |
||||
Table 4: Prevalence of Cryptosporidium spp., Giardia spp. and Entameba spp. infection among breeders in regards to contact with camels according to age of breeders
|
Sex |
Total Breeders |
Cryptosporidium spp. |
Giardia spp. |
Entameba spp. |
|||
|
No. of Positive |
(%) |
No. of positive |
(%) |
No. of positive |
(%) |
||
|
<30 |
11 |
6 |
54.54 |
1 |
9.09 |
1 |
9.09 |
|
30-60 |
14 |
7 |
50 |
4 |
28.75 |
3 |
21.42 |
|
Chi sq |
0.05 |
1.46 |
0.69 |
||||
|
P value |
0.82 |
0.22 |
0.40 |
||||
Table 5: Comparison of infection rate between camels and their breeders
|
Sex |
Total No. |
Cryptosporidium spp. |
Giardia spp. |
Entameba spp. |
|||
|
No. of Positive |
(%) |
No. of positive |
(%) |
No. of positive |
(%) |
||
|
Camels |
100 |
61 |
61 |
24 |
24 |
20 |
20 |
|
camel's breeders |
25 |
14 |
56 |
5 |
20 |
4 |
16 |
|
Chi sq |
0.20 |
0.17 |
0.20 |
||||
|
P value |
0.64 |
0.67 |
0.64 |
||||
The probability less than 0.01 or 0.05 was significant (P<0.05) (P<0.01)
(16%) and Giardia spp. (20%). All differences between males and females across all types of protozoa were not significant.
Among Camel’s Breeders according to Age of Breeders
The results obtained in Table 4 the highest infection rate of Cryptosporidium spp. was 54.54% at age <30years while the highest infection rates of Giardia spp. and Entameba spp. Were 28.75% and 21.42% in age 30-60 years, respectively. Statistical analysis shows that the differences in prevalence among age categories across the three types of protozoa were not significant.
The Table 3 and 4 show that there were no effects of the age and sex on the prevalence of Cryptosporidium spp. of camels breeders. These results were in agreement with Sazmand et al. (2012) in Iran who recorded the prevalence of Cryptosporidium spp. in camel’s breeders as 24% this percentage was lower than prevalence of Cryptosporidium spp. found in our study which was 56% in camel’s breeders, while the prevalence of Giardia spp. and Entamoeba spp. was 20% and 16%, respectively. These percentages were higher than percentage 6.6% and 1.7% reported by Mahdi and Ali (2002) for animals handlers and non-animal handlers respectively. On the other hand, Entamoeba in the animals handlers was 7 out of 60 (11.6%) while in non-animal handlers was 8 out of 175 (4.5%).
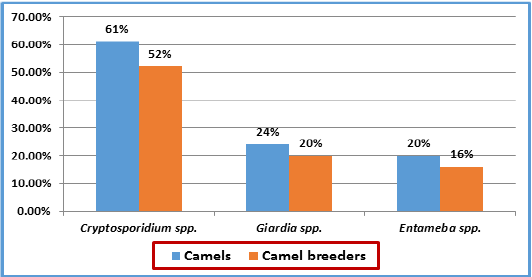
Comparison of Infection Rate between Camels and their Breeders
Results in Table 5 show that the infection rates in camel’s breeders and camels were nearly close with non- significant differences across the three types of protozoa. The trend of infection of the three types of protozoa in camel’s breeders is parallel to the trend of infection rates in camels (Figure 1). This could represent evidence about the mutual infection between camel’s breeders and camels.
CONCLUSION
The study conclude the high prevalence values of the intestinal protozoa were detected in camels and camel’s breeders. The prevalence values seems to be parallel between camels and camels breeders who in touch with camels. This could be an evidence of the association infection between camels and camel’s breeders.
ACKNOWLEDGMENT
The authors would like to thank the Department of Zoonotic Unit and Department of Parasitology, Faculty of Veterinary Medicine, University of Baghdad, Iraq for their advice and support.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHORS CONTRIBUTION
All authors contributed equally in all details of this manuscript.
REFERENCES