Advances in Animal and Veterinary Sciences
Case Report
A Fatal Case of Toad (Buffo Melanostictus) Intoxication in a German Shepherd Dog and Review of Literature
Fizzah Laeeq Lodhi*
Faculty of Veterinary Sciences (FVS), University of Agriculture Faisalabad (UAF)-38040, Punjab, Pakistan.
Abstract | In the present communication, an unprecedented and fatal case of Buffo melanostictus intoxication in a German Shepherd dog, presented to Veterinary Medical Teaching Hospital, University of Agriculture Faisalabad Pakistan, for the treatment of profuse salivation, prostration, excessive vocalization, vomiting, hemorrhagic diarrhea and haematuria after accidently chewing a toad (Buffo melanostictus) is put on the record. The clinical examination revealed normal rectal temperature, tachypnea, arrhythmic tachycardia, deep red mucous membranes, elevated capillary refill time and severe neurological signs. Hematological analysis indicated decreased level of hemoglobin, packed cell volume, total red blood cells, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration along with leukocytosis, lymphopenia, neutrophilia, eosinophilia and monocytosis. The significant findings of biochemical panel were including, increased level of blood urea nitrogen, creatinine, liver enzymes, total proteinsand serum potassium. A symptomatic treatment regime was adopted but the patient died on 2nd day post treatment. Diagnosis of toad poisoning was arrived on the basis of pathognomonic clinical signs, hemato-biochemical findings, nevertheless the identification of chewed toad further supported the diagnosis. Additionally, treatment regime, management and known factors influencing toad poisoning along with prognosis are also reviewed.
Keywords | Buffo melanostictus, German Shepherd, Hematology, Biochemical analysis, Chewing, Pakistan.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | January 20, 2018; Accepted | March 12, 2018; Published | April 15, 2018
*Correspondence | Fizzah Laeeq Lodhi, Faculty of Veterinary Sciences (FVS), University of Agriculture Faisalabad (UAF)-38040, Punjab, Pakistan; Email: [email protected]
Citation | Lodhi FL (2018). A fatal case of toad (buffo melanostictus) intoxication in a german shepherd dog and review of literature. Adv. Anim. Vet. Sci. 6(4): 156-160.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.4.156.160
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Lodhi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Among amphibians, toads are the most invasive pest on the earth encountered by humans and companion animals. Toads belonging to order Anura, family Bufonidae and genus Bufo are preferably found in the tropical and subtropical regions of the world (Camplesi et al., 2010). Amphibians are not outfitted for alacrity, nor do they have any munition; as such they rely primarily on chemical defense to dissuade their predators. Toads are often regarded as poisonous animals instead of venomous owing to the fact that they are devoid of inoculating tools (Camplesi et al., 2010). Mostly the glandular skin secretions of the toad’s belonging to genus Bufo are highly poisonous; nevertheless, the poison has also been isolated from the eggs and plasma of few toads (Yei and Deng, 1993). The parotid glands situated bilaterally in the post-orbital region of the toads are chief site of production and storage of the poison (Eubig, 2001). Dogs are more prone to be victimized probably due to their inquisitive nature, being enticed by the toad’s dawdling movements, particularly in the early morning and night (MacDonald, 1990). Inadvertent toad poisoning may take place in dogs, particularly in the environs of streams, lakes, rivers and reservoirs, which are the natural habitats of toads (Guscio et al., 2008). Dogs become poisoned by mouthing, chewing or ingesting the toad, which allows the penetration and subsequent absorption of the poison in the oral and digestive tract mucosa, facilitating the expression of its toxic effects throughout the body.In the present communication a fatal case of Buffo (B,) melanostictus intoxication in a German shepherd dog is put on the record.
Case Presentation
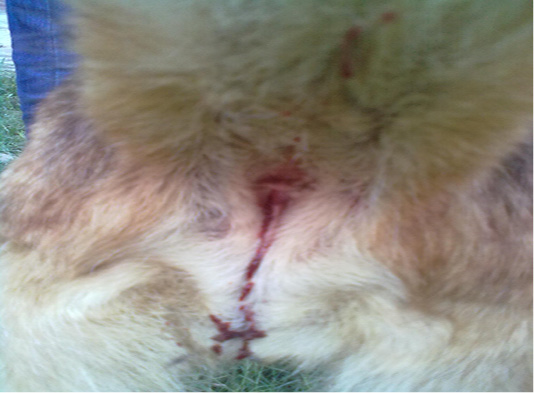
A 3-years old intact female German shepherd dog weighing 20 kg was presented to Veterinary Medical Teaching Hospital (VMTH), University of Agriculture Faisalabad, Pakistan within 3 hours of profuse salivation, prostration, vocalization, vomiting, hemorrhagic diarrhea and haematuria, (Figure 1) immediately after mouthing and chewing a toad in the early morning, which was witnessed by the guardian during a close supervision. The dead toad was collected and latter on identified as B.melanostictus. Aforementioned to this, the dog had no major medical anamnesis and had always been apparently healthy. Vaccination of the dog was current and included those against canine distemper, parvo, rabies, infectious hepatitis, leptospirosis and adeno virus (Hexadog®).The dog was on parasitic control program comprising of commercially available suspension product containing pyrantel pamoate (Combantrin®).The dog was individually housed in a kennel and had free access to lawn. At VMTH, the dog underwent routine physical, clinical, radiological, neurological and hemato-biochemical examination. Clinical examination revealed normal rectal temperature (39 o C), tachypnea (50 breaths/ minute), arrhythmic tachycardia (130 beats/ minute) and deep red mucous membranes with capillary refill time of 5 second. Ophthalmic examination indicated normal palpebral, pupillary light reflex and menace response in both eyes. The findings of urinalysis and radiographical examination were unrewarding. Acute neurological impairments including ataxia, head shaking and interpolating seizures were remarkable findings of neurological examination. Decreased level of hemoglobin (Hb) packed cell volume (PCV), total red blood cells (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) along with leukocytosis, lymphopenia, neutrophilia, eosinophilia and monocytosis were the significant findings of hematological (CBC) analysis (Table 1). The results of biochemical panel were remarkable including elevated level of blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total proteins (TP) especially globulin and serum potassium (Table 2). However, a minimally dropped level of serum sodium and chloride ions was observed. A symptomatic treatment encompassing fluid therapy (Infusion 0.9% NaCl; 40ml/kg, IV, q12h), metoclopramide (Injection Maxilon®, 0.5mg/kg, q8h, SC), rantidine (Injection Zentac®; 1.5mg/kg, q8h, SC), metronidazole (Infusion Flagyl®; 25mg/kg, q12h, IV), ceftrioxone sodium (Injection Oxidil®; 20mg/kg, q12h, IV), furosemide (Injection Lasix®; 2mg/kg; q12h, IV), lidocaine (Injection Xylocaine®; 2 mg/kg, q24h, IV), diazepam (Injection Valium®; 0.4 mg/kg, q12h, IV) and activated charcoal (1 gram/kg; SID PO) was instituted to ameliorate the poisonous effect. The patient was kept under observation for 5 hours at VMTH and discharged
Table 1: Hematological values of a female German shepherd suffering from Buffo melanostictus toxicosis.
|
Parameters
|
Values taken before and after initiation of treatment | ||
| Day -1 | Day-2 | Reference Values * | |
|
Red blood cells (RBC) ( x 1012g/L) |
4.2 | 3.2 | 5.5-8.5 |
| Packed cell volume (PCV) (%) | 32 | 27 | 37-55 |
| Hemoglobin (Hgb) (g/dL) | 7 | 4 | 12-18 |
| Mean corpuscular volume (MCV) (fL) | 77 | 84 | 60-77 |
| Mean corpuscular hemoglobin (MCH) (pg) | 16.6 | 12.5 | 19.5-24.5 |
| Mean corpuscular hemoglobin concentration (MCHC) (x 10 g/L) | 21.9 | 14.8 | 32-36 |
|
White blood cells (WBC) (x 109/L) |
25 | 31 | 6-17 |
| Neutrophils (%) | 74.2 | 73.4 | 60-70 |
| Lymphocytes (%) | 1.4 | 1.3 | 12-30 |
| Monocytes (%) | 11.2 | 3.5 | 4-10 |
| Basophils (%) | 1 | 8.5 | Rare |
| Eosinophils (%) | 12.2 | 14.3 |
2-10 |
Table 2: Serum biochemistry values of a female German shepherd suffering Buffo melanostictus toxicosis.
|
Parameters
|
Values taken before and after initiation of treatment | |||
| Day-1 | Day-2 | Reference Values* | ||
|
Alanine Aminotransferase (u/l) |
100 |
122 |
8.2-57 | |
|
Alkaline Phosphatase (u/l) |
110 | 129 | 10.6-101 | |
| Gamma-Glutamyl Transferase | ||||
| Total Protein | ||||
| Creatinine (mg/dL) | 2.5 | 3.2 | 0.5-1.6 | |
| Blood Urea Nitrogen (mg/dL) | 38 | 52 | 8.8-26 | |
| Serum Potassium | ||||
| Serum Sodium | ||||
| Serum Chloride | ||||
with a little clinical progress. On the following day, the dog was presented in comatosed position manifesting sever tachypnea (80 breaths/ minute), arrhythmic tachycardia (160 beats/ minute), intermittent seizures and deteriorating picture of hemato-biochemical profile especially hyperkalaemia (Table 1 and 2). Before any treatment was suggested the dog deceased. Necropsy was planned in order to investigate postmortem lesions associated with B. stomaticus poisoning but the guardian was reluctant. Diagnosis of B. melanostictus poisoning was arrived on the basis of anamnesis and identification of wrongdoer along with clinical signs and hemato-biochemical findings consistent with toad poisoning.
Discussion
Toxicity remains primarily a veterinary malady, although the documented reports of human and veterinary toxicity caused by toads are scarce (Allen and Neill, 1956). Generally, Pakistan is categorized as an amphibian-poor state because of its existing arid climatic conditions. There are eight existing species of the genus Bufo in Pakistan. Among these B. melanostictus are essentially nocturnal and the habitats where they are frequently found include woods, piles of bricks, slits and walls. Previous investigations had revealed that various pharmacologically potent and bioactive substances with hypotensive, lethal, hypertensive, cardiotoxic, neurotoxic, hemolytic and sleep inducing properties are present in the venom of B. melanostictus (Das et al., 2000). Members of the family Bufonidae exude a wide variety of compounds belonging to different chemical classes with diverse pharmacological activities. Many of these substances could serve the toads in defense as chemical irritants and secondary to a physiological task. The chemical analysis of the venom of various Bufo sp. had enabled the researchers to characterize more than 100 chemical constituents of the venom. The major active substances of the venom can be classified into two groups including biogenic amines and steroid derivatives (Peterson and Roberts, 2006). Bufotenines (serotonin and 5-hydroxytryptophan), bufotyonines, dihydro-butenines and catecholamines (dopamine, epinephrine and norepinephrine) can be highlighted under the category of biogenic amines owing to their toxic relevance (Peterson and Roberts, 2006; Reeves, 2004). Bufotenines in combination with catecholamines are responsible for various gastrointestinal and neurological effects of Bufo toxins (Eubig, 2001).
Among the steroid derivatives, digitalis like substances including bufotoxins and bufodienolids are present (Eubig, 2001; Barbosa et al., 2009; Kuo et al., 2009; Camplesi et al., 2010). Bufadienolides are the most meticulously investigated constituent of the venom, not only due to its chemical diversity, but also with respect to their bioactivity (Goa et al., 2010). These steroid derivatives inhibit the functioning of the Na+-K+ ATPase activity in myocardial cell membrane, resulting in increased calcium influx and blockage of sodium channels in myocardial cells and eventually cardiac arrhythmias such as ventricular fibrillation are observed (Eubig, 2001). The results of present communication had corroborated the findings of previous studies, reporting elevated level of BUN, ALT, ALP, GGT, TP and K in the patients affected with Bufosp. toxicosis (Eubig, 2001; Peterson and Roberts, 2006; Barbosa et al., 2009; Kuo et al., 2009; Camplesi et al., 2010). Additionally the decreased level of Hb, PCV, RBC, serum Na+ and Cl+ are in consistent with the findings of Palumbo (1975).
Several factors including toad’s specie, victim’s age and body weight, habitat, concurrent disease and exposure time are incriminated to forecast the clinical outcome (Sakate, 2001; Barbosa et al., 2009; Camplesi et al., 2010). Smaller patients are often presented with sever clinical signs of toxicosis. It has been estimated that a dose of 1 mg/kg is sufficient enough for the manifestation of severe clinical signs (Eubig, 2001), however, some studies have mentioned that an oral dose of 0.1 g of venom is lethal for a dog (Reeves, 2004). In relation to diagnosis, therapeutic orientation and prognosis, clinical signs can be categorized into three group’s viz., mild, moderate and severe. Mild cases are characterized by the irritation of oral mucous membrane and sialorrhea. Besides aforementioned signs, animals with moderate cases of toxicity are presented withdepression, vomiting, ataxia, fecal and urinary incontinence, atypical cardiac rhythms and neurological disturbances. In severe cases, in addition to the abovementioned signs, abdominal pain, diarrhea, pokerfaced pupils, cyanosis, seizures, pulmonary edema, nystagmus, opisthotonus and death might take place within 15 minutes (McFarland, 1999; Reeves, 2004). Electrocardiographic changes associated with Bufo toxicosis comprise ongoing deterioration of typical pattern with progressive negative ventricular deflections and ventricular rhythm along with negative and deep QRS complexes (Barbosa et al., 2009). In some cases progressive muscular paralysis, excessive vocalization and blindness has also been documented (Sakate, 2002). The documented clinical signs of the present case study are in consistent with severe form of the toxicosis as observed previously (Barbosa et al., 2009).
On the basis of clinical signs, recent exposure to Bufo sp. and anamnesis, toxicity should be differentiated from all those disorders manifesting similar clinical picture. Among these some ataxic conditions (peripheral and central vestibular diseases), trauma (road accident), heat stroke, seizure disorders (idiopathic epilepsy, internal masses and inflammatory mengioencephalitis) insecticides (organophosphorus, pyrethroids, carbamates and chlorinated hydrocarbons) and caustic agent poisoning are top ranking (Eubig, 2001; Peterson and Robertson, 2006). Additionally, ingestion of some outdoor toxic plants especially oleander (Nerium oleander), foxglove (Digitalis purpurea) and rhododendron (Rhododendron sp.) should also be ruled out. Revelation to some drugs particularly sympathomimetics, β-blockers, β-agonists, methylxanthine and various antidepressants may present similar clinical findings (Eubig, 2001). In the present case study all the aforementioned conditions were excluded on the basis of witnessed exposure to B.melanostictus, although identification of the dead doer further supported the diagnosis.
Medical management of the victimized patient depends upon the nature of exposure and underlying clinical signs. In mild cases where the toad has been merely mouthed and released, on-the-spot decontamination and lavage of the oral cavity with copious amount of water is recommended. If the toad has been chewed or swallowed aggressive intervention along with cardiac and neurological monitoring is warranted in order to stabilize the patient. Emesis could be induced in those patients, where no sign beyond hypersalivationhas been observed. If the patient is displaying severe clinical signs following ingestion of toad than endoscopically or surgically removal of the toad is warranted after stabilizing the patient (Gwaltney-Brand et al., 2007;Eubig, 2001). Alternatively, multiple dose of activated charcoal along with a cathartic agent could be administered, although the clinical efficacy of activated charcoal for absorbing Bufo toxins has not specifically been assessed (Eubig, 2001). Patients displaying severe clinical toxicosis should be immediately subjected to intensive symptomatic treatment. Special attention should be paid to monitor electrocardiogram if cardiac abnormalities are detected. Cardiac arrhythmias especially bradycardia and tachycardia should be addressed using atropineand propanolol, respectively. Fluid therapy should be a part of treatment regime to aid in cardiovascular support (Eubig, 2001; Gwaltney-Brand et al., 2007). The use of atropine to control hypersalivation and lung secretions is controversial since it reduces the elimination of the poison through saliva (Barbosa et al., 2009). Therefore, in the present study atropine was not administered. The majority of patients affected from Bufo toad toxicosis feature a significantly elevated level of serum potassium, which is frequently associated with a guarded prognosis and a rather high mortality rate. An elevated serum potassium level features prognostic insinuation for individual’s purportedly falling victim to Bufo toxicosis, particularly if hyperkalaemia develops, such that succeeding treatment needs to be more belligerent than might initially be deemed essential, in order to avert mortality (Kuo et al., 2007).Patient experiencing severe neurological signs should be immediately subjected to diazepam or any barbiturate therapy (Eubig, 2001). The therapeutic efficacy of digoxin-specific antigen binding fragments have proven to be beneficial in the patients exhibiting severe hyperkalemia along with acute neurological and cardiac signs unresponsive to routine therapy (Gwaltney-Brand et al., 2007). However, this drug is expensive and might not be readily available, rendering its use unfeasible in veterinary medicine.
The relative venom potency of various Bufo sp. especially B. marinus, B. blombergi, B. alvarius and B. regularis has already been documented (Eubig, 2001; Barbosa et al., 2009), nevertheless, the subject case study is a solitary report documenting the severe toxicosis in a dog following exposure to B.melanostictus which needs to be further investigated.
Acknowledgements
The author would like to acknowledge owner of the dog for giving authorization to publish this report.
Conflict of interest
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article
Authors Contribution
Fizzah Laeeq Lodhi wrote the manuscript as well as handled the case.
References