Advances in Animal and Veterinary Sciences
Research Article
Potential of ‘Goat Based Vaccine’ using ‘India Bison Biotype’ of Mycobacterium avium Subspecies paratuberculosis in Salvaging a Dairy Farm Consisting of High Yielding Holstein Fresian Cows from Devastation and Closure Due to Outbreak of Bovine Johne’s Disease in Northern India
Krishan Dutta Rawat1, Sarjeet Chaudhary2, Saurabh Gupta1, Kundan Kumar Chaubey1, Sujata Jayaraman3, Naveen Kumar1, Jagdip Singh Sohal3, Tarun Kumar Sachan1, Kuldeep Dhama4, Ruchi Tiwari5, Shoor Vir Singh1
1Animal Health Division, Central Institute for Research on Goats, Makhdoom, Farah-281122, Mathura, Uttar Pradesh; 2Veterinary Officer, State Animal Husbandry Department, Dist. Alwar, Rajasthan; 3Amity Institute of Microbial Technology, Amity University Rajasthan, Kant Kalwar, NH 11C Delhi-Jaipur Highway-303 002, Jaipur, Rajasthan; 4Department of Pathology, Indian Veterinary Research Institute, Izatnagar-243 122, Uttar Pradesh. 5Department of Veterinary Microbiology and Immunology, Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishwa Vidyalaya Evam Go-Anusandhan Sansthan (DUVASU), Mathura – 281001, Uttar Pradesh, India.
Abstract |Present study used ‘goat based’ Johne’s disease vaccine to save cows from imminent closure and restored health (weakness and emaciation) and productivity (milk production) in a dairy farm consisting of high yielding Holstein Friesian cows in the Alwar district of Rajasthan (India) in the year 2012. High yielding Holstein Fresian cows being susceptible to diseases including Mycobacterium avium subspecies paratuberculosis suffered from an outbreak of JD. Affected cows exhibited significant reduction in milk yield (p<0.05). Other losses were due to forced removal, mortality and reduced productivity (infertility, stunted growth etc.). Heifers showed progressive weakness, stunting and delay in onset of heat (sexual maturity). ‘Indigenous vaccine’ helped not only in improving physical condition and weakness but also restored the productivity and milk yield. Cows recorded increasing trend in milk yield and at 90 days post vaccination milk production improved by >2.1 litres/day (total increase of 49 litres/day). Shedding of the MAP bacilli in feces of vaccinated cows was also reduced drastically and was completely stopped in some of the cows. Study showed that JD outbreak caused heavy losses in H/F breed of cows by way of early removal of cows, reduced fertility, drop in milk production. ‘Indigenous goat based vaccine’ not only restored health and productivity of affected cows but cured these cows of Johne’s disease within 10 months period. Therefore, this vaccine can be used to restore productivity of large number of low and unproductive cows in the country due to ban on cow slaughter.
Keywords | Economic losses, Clinical Johne’s disease, Indigenous vaccine, Paratuberculosis, ELISA, Blood PCR, Microscopy
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 03, 2014; Revised | November 18, 2014; Accepted | November 20, 2014; Published | December 01, 2014
*Correspondence | Shoor Vir Singh, Central Institute for Research on Goats, Makhdoom, Uttar Pradesh, India; Email: [email protected]
Citation | Rawat KD, Chaudhary S, Gupta S, Chaubey KK, Jayaraman S, Kumar N, Sohal JS, Sachan TK, Dhama K, Singh SV (2014). Potential of ‘goat based vaccine’ using ‘India bison biotype’ of Mycobacterium avium subspecies paratuberculosis in salvaging a dairy farm consisting of high yielding Holstein Fresian cows from devastation and closure due to outbreak of bovine Johne’s disease in Northern India. Adv. Anim. Vet. Sci. 2 (12): 638-646.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.12.638.646
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Rawat et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Johne’s disease (JD) caused by Mycobacterium avium subspecies paratuberculosis (MAP) is reported endemic in domestic livestock population of countries where investigated (Verma, 2013; Ayele et al., 2001) including India (Singh et al., 2014a). However, incidence of JD has shown increasing trend in the country (Singh et al., 2014b), despite heavy slaughter of goats, sheep and buffaloes. Incidence of JD in cattle population of the country has been reported to be highest, primarily due to ban on ‘cow slaughter’. Since goats, sheep and buffaloes can be salvaged for meat production, their population has increased consistently in last 65 years, whereas, population of cows has shown decreasing trend (FAO, 2013). High incidence of JD is primarily responsible for ‘low per animal productivity in native breeds of domestic livestock’. Unprecedented increase in human population led to high demand of milk and milk products. To meet this challenge and to boost per cow productivity of native breeds, cross breeding programme with high yielding temperate breeds’ like Holstein Frisian (H/F) and Jersey were initiated in 1960s. Though H/F cows from various health problems, however this is the first report of the outbreak of JD in dairy cattle herd. (Singh et al., 2014a). In India bovine JD testing was initiated in 1950s, but due to ban on cow slaughter, problems associated with maintenance of positive cows and absence of National programs for the control, population of low and un-productive cows has increased. Singh et al. (2014a) reported a rare outbreak of JD in high yielding H/F cows in a dairy farm located in Alwar district of Rajasthan. Dairy farm reported heavy economic losses due to JD. Present study first time evaluated goat based ‘Indigenous Vaccine’ to salvage dairy farm consisting of 71 cows and followers of Holstein Frisian breed from imminent closure due to outbreak of Johne’s disease. Cows were vaccinated for ‘therapeutic management’ of JD and were monitored for improvements in the dairy farm on the basis of sero-conversion, shedding of bacilli in faeces, physical, health and production in 10month period after vaccination.
Materials and Methods
Farm History and Outbreak
A dairy farm consisting of H/F cows was established in June 2011 as commercial dairy farm for the livelihood by an entreprener in a new place (village Dalalpur PO- Shahpur) Alwar district of Rajasthan by purchasing cows from different parts of the country. Semen of H/F bulls was used for breeding from a semen bank (Bassi, Jaipur, Rajasthan) to maintain high yielding status of cows. At the time of Johne’s disease outbreak, there were 71 cows [19 (0-18m), 11 (18-30m) and 41 (>30m)] cows in the dairy farm. Clinically cows were unthrifty and poor in body condition and suffered with problems of progressive weight loss and weakness without diarrhea along with sharp decline in milk production during our first visit to dairy farm in October, 2013 to investigate the outbreak. Fecal, blood, serum and milk samples of 35 cows (26 adult and 9 calves) were collected for screening of MAP infection.
Nutrition and Management
Cows were housed (intensive management), maintained under optimum nutrition and were provided cultivated green fodder, conserved forage, crop residues and concentrates in hygienic conditions. Cows were provided protection against extreme cold and hot weather.
Profile of Losses in Production after Outbreak of JD
Losses due to outbreak of JD were estimated precisely across the herd. Cost of cows at the time of death was taken equivalent to the cost of replacement. As per the owner of dairy farm, cost of a healthy cow averaged approximately Rs. 40,000.0 in Rajasthan, in Oct., 2013. Average replacement value of the cows was also taken as the slaughter value of cows, though cow slaughter is banned in India. Carcass value of dead cows was nil.
Milk Production
Losses in milk production were recorded and milk yield of 31 lactating cows before and after outbreak of Johne’s disease were compared. Significant reduction in milk yield was observed for cows after outbreak of JD. Average reduction in milk yield 305 days (10 months) of lactation were recorded.
Mortality and Morbidity
Mortality, morbidity and gross lesions at necropsy were documented by the consulting local veterinary practitioner.
Forced Removal
Cows suffering with advance symptoms of JD and positive for MAP infection were removed from herd to protect others cows.
Reproductive Losses
Reproductive losses in the herd were estimated on the basis of the conception rate in normal and healthy healthy cows and date of first conception was 24 months of age in the H/F cows. Cost of daily feeding and management during extended time period of conception, calving and calving interval were used to estimate reproductive losses, Extra cost of maintenance, treatment of sick and management of affected animals was also included in losses.
Vaccine, Vaccination and Monitoring of Vaccine response
‘Indigenous vaccine’ developed by Singh et al. (2007a), using novel, native, highly pathogenic, and genetically characterized ‘S 5’ strain of ‘Indian Bison Type’ biotype of MAP was isolated from a clinically sick goat (goat based) that died of JD at one of the goat farms located at Central Institute on Research on Goats (CIRG). Vaccine contained 2.5 mg (dried weight) of heat inactivated native strain (‘S 5’) of MAP bacilli (approximately 12 x 108 bacilli) suspended in one milliliter of Aluminum hydroxide gel (CZ Veterinaria, Spain). All animals above 4 months of age were vaccinated with 2 ml of ‘indigenous vaccine’ subcutaneously in the neck region behind the ear. Vaccinated cows were monitored for study period (10 months) at different time intervals (0, 60, 120 and 180 days post vaccination) for immune response by indigenous ELISA (Singh et al., 2009), shedding of MAP in faeces by microscopy (Singh et al. 2014b) and changes in production parameters. Overall improvements on the basis of health (morbidity), mortality, production (reproductive efficiency, milk production), physical and clinical conditions (weakness, diarrhea, skin coat) were measured. PCR on blood samples was performed as per Singh et al. (2014b).
Results
Profile of Production Losses after Outbreak of Johne’s Disease
High milk yielding cows of Holstein Friesian (H/F) breed suffered with severe outbreak of Johne’s disease within 2 years of establishment of the dairy farm. On the basis of clinical symptoms and losses in milk yield local consulting veterinarian suspected outbreak of JD, which was confirmed using multiple diagnostic tests (Singh et al., 2014a). Screening of fecal, blood, serum and milk samples of 35 cows (26 adult and 9 calves) showed, 68.5, 92.3, 40.8 and 35.7% animals were positive for Mycobacterium avium subspecies paratuberculosis infection using fecal microscopy, serum and milk ELISA and IS900 blood PCR, respectively. Cows exhibited marked improvements after vaccination in all the parameters of health and production, without any changes in nutrition regimen and management.
Cows in both lactating and in other age groups exhibited marked improvement in physical condition and in clinical symptoms of weakness and loss of body condition, within 90 days of vaccination. Cows became active and alert, regained shining of skin coat and improvement in physical appearance. None of the cows exhibited diarrhea during outbreak of JD. Growth rate of young cows showed increasing trend and cows started recovering from weakness and wasting. Some of the cows developed small nodule at the site of vaccination (take), which regressed slowly (8-10 weeks). Total losses due to outbreak of JD in the dairy farm were Rs. 5,67,176.0. Losses due to reduction in milk production of 23 high milk yielding H/F cows were 23.1% (Rs. 1,31,376.0) in 305 days of lactation. There was 5.0% mortality in cows and was higher in JD positive cows as compared to JD negative cows. Gross pathological lesions of thickening of intestinal mucosa and enlargement of lymph nodes were recorded by consulting veterinarian. In the present study 7 cows infected with MAP neither conceived nor showed symptoms of heat upto and beyond the age of 30 months. Losses also included extra cost of maintenance, treatment and management of JD affected cows. Losses due to forced removal of 4 extremely weak were 29.4% (Rs.1,67,000.0) in the year under study. Annual losses due to mortality and reproductive disorders were 18.5% (Rs. 1,05,000.0) and 28.0% (Rs. 1,63,800.0), respectively.
Improvement in Milk Production
Average milk production of the dairy herd was reduced from 407 litres/cow to 183 litres/cow in 305 days of lactation after the outbreak of JD. The decline of 224 litres /cow in 305 days of lactation in terms of value was Rs. 5712 /cow (average price of milk in this period was Rs. 25.50/litre)). Of the total 31 lactating cows monitored for loss in milk production, the average reduction of 224 litters/cow was Rs. 1,31,376.0 in 23 JD positive cows. Losses due to reduction in milk yield of cows negative for JD were not significant (p>0.05). In comparison to healthy cows infected cows suffered from significant reduction in milk yield (p<0.05) in the lactation period of 305 days except first month of lactation (Figure 1). After JD vaccination cows started exhibiting increasing trend (>2.1 litres/day/cow) (Figure 1) in milk production and three months post vaccination total milk production 23 cows increased by 49 litres/day (Table 3). However, there was no effect in milk yield of healthy cows. Of the total losses (Rs. 5,67,176.0) due to outbreak of JD in the dairy farm, losses due to reduction in milk yield of 23 lactating cows affected with JD were Rs. 1,31,376.0 (23.1%).
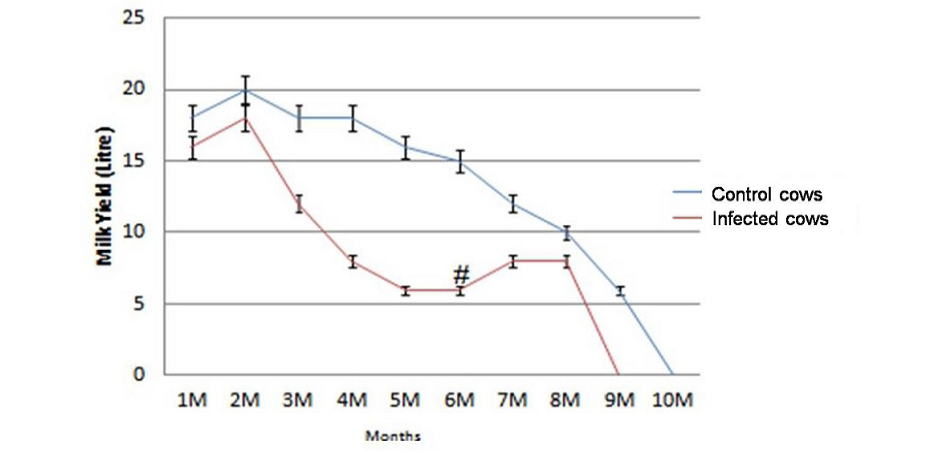
Figure 1: Significant reduction (p<0.05) in total milk yield of infected cows (n=23) in comparison with healthy control cows (n=8) and increase in milk production at 6th month (#) post vaccination.
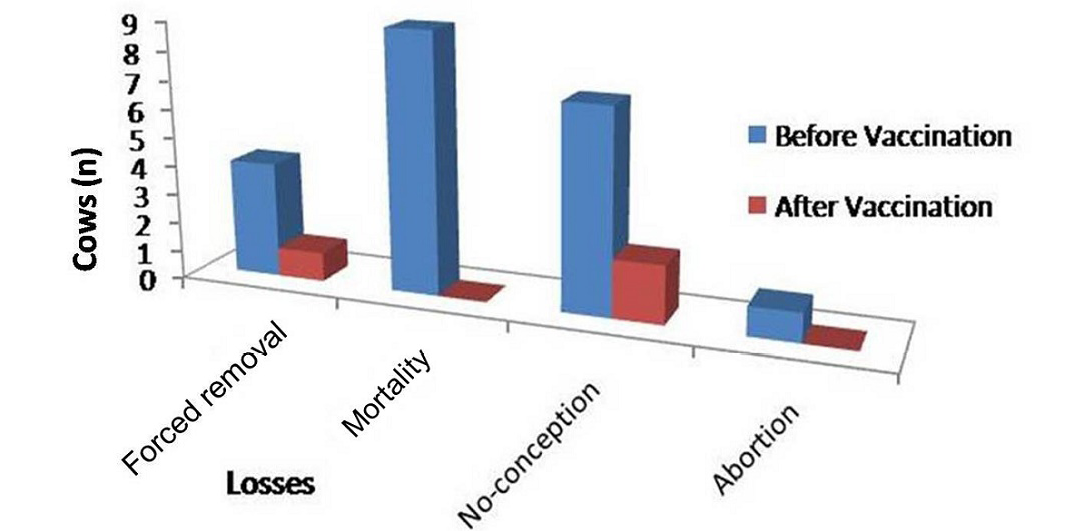
Figure 2: Improvement in dairy cows in forced removal, mortality, no-conception and abortion following Johne’s disease vaccination at Alwar (Yadu Dairy Farm)

Figure 3: Representative photographs showing physical appearance of cattle at different days post vaccination
Improvements in Mortality and Morbidity
Physically at 180 DPV infected cows regained good health and shining on skin and cows recovered from weakness (Figure 2 and Figure 3). Conception rate was improved after vaccination at different time points (seven cows conceived at 32 months of age), both mortality and abortions were reduced by 100%, whereas forced removal was reduced by 75% at 180DPV (Figure 3). This status of dairy cows was maintained up to 360 DPV and beyond.
Reduction in Shedding of MAP in Feces and Blood PCR
Screening of fecal samples at different time points (0, 60, 120 and 180 DPV) showed that there was marked reduction in shedding of MAP bacilli in faeces. Shedding of MAP was reduced by 35.6 and 46.6% in faeces of cows at 60 and 180 DPV, respectively. IS900 blood PCR of the cows positive before vaccination became negative (100% reduction) at 60DPV and this conditions was maintained at 120 and 180 DPV (Table 1).
Table 1: Monitoring of vaccine response in dairy cattle by fecal microscopy (MAP shedding status), indigenous ELISA kit (sero-conversion) and blood PCR (status of MAP infection) at 0, 60, 120 and 180 DPV
|
Tests |
Group |
0 DPV |
60 DPV |
120 DPV |
180 DPV |
||||
|
n |
Positive n (%) |
n |
Positive n (%) |
n |
Positive n (%) |
n |
Positive n (%) |
||
|
Microscopy |
Vaccinated |
60 |
40 (66.6) |
31 |
7 (31) |
30 |
6 (20) |
30 |
6 (20) |
|
ELISA |
Vaccinated |
25 |
22 (88) |
25 |
22 (88) |
25 |
20 (80) |
10 |
8 (80) |
|
Blood PCR |
Vaccinated |
20 |
8 (40) |
25 |
0 (0) |
25 |
0 (0) |
10 |
0 (0) |
Humoral Immune Response (ELISA)
After vaccination higher sero-conversion rates were seen in vaccinated cows as compared to non vaccinated cows (Table 2). Percent of cows sero-converted remained higher in ‘vaccinated group’ than in ‘control group’ at all post vaccination sampling intervals. Almost all the cows in vaccinated groups became sero-positive (sero-converted) at 60 DPV significantly (p<0.05) as compared to the status at 0 DPV (Table 3).
Table 2: Average S/P ratios in vaccinated and control cows from 0 to 180 days post vaccination
|
Groups |
Cows (n) |
S/P ratios (Average) |
|||
|
0DPV |
60DPV |
120DPV |
180DPV |
||
|
Vaccinated |
23 |
0.539±0.03 |
0.831*±0.06 |
0.722±0.03 |
0.721±0.05 |
|
Control |
8 |
0.259±0.04 |
0.673±0.03 |
0.617±0.05 |
0.691±0.07 |
* S/P ratio was significant (p <0.05) between 0 and 60 DPV in vaccinated group
Table 3: Losses in milk production due to outbreak of Johne’s disease in a H/F dairy farm, Alwar (Rajasthan)
|
Cows in-milk (March 2013 to February 2014) 305 days of lactation |
Average milk production of 31 cows (in litres) per day |
|||
|
Before outbreak (March 2013 to August 2013) |
After outbreak (September 2013 to February 2014) |
Reduction in milk (March 2013 to February 2014) |
Total milk increased after vaccination |
|
|
n=31 |
562 ± 29 |
338 ± 22 |
224 ± 17 |
49 ±3 |
|
Healthy (8) |
155 ± 5 |
155 ± 5 |
No effect |
No effect |
|
Infected (23) |
407 ± 24 |
183 ± 17 |
224 ± 17 |
49 ±3 |
H/F- Holstein Frisian cows, 31 cows includes 8 apparently healthy and 23 MAP infected cows
Discussion
Rising cost of feed, poor disease management, reduced productivity and low or zero salvage value is hallmark of domestic livestock production system (Richardson and More 2009). Johne’s disease is the major infectious disease of domestic livestock and is directly correlated with the low productivity of native breeds. In India, Johne’s disease is endemic in large population of domestic livestock (Singh et al., 2014c). High producing animals are being removed regularly, consequently the economic losses increased significantly (Beaudeau et al., 2007). In the present study dairy farm at Alwar suffered from huge economic losses due to JD (total losses Rs. 56,776.0) from reproductive disorders mainly sub-fertility (Rs. 23400.0/cow/year), forced removal (41,750.0/cow/year), milk (Rs. 5,712.0/cow/year) and mortality (Rs. 11,666.0/cow/year) within two years of establishment of the dairy farm wherein outbreak of JD was reported and confirmed (Singh et al., 2014a). There is no comparable study reporting losses and outbreak due to JD in cattle dairy farms. However, VinodhKumar et al. (2013) reported losses to the tune of Rs. 1840/sheep/year. Effect of MAP sero-prevalence showed a co-relation between MAP infection and milk production (Tiwari et al., 2007).
Multiple tests are required for the screening of animals exposed to MAP at early stage, in order to reduce disease burden and transmission. Milk (Singh et al., 2007b) and serum (Singh et al., 2009) based ‘ELISA kits’ and ‘indigenous vaccine’ have been developed
for the diagnosis and control of JD in goats and sheep using well characterized strain ‘S 5’ of native ‘Indian bison type’ biotype. ‘Indigenous vaccine’ developed showed remarkable therapeutic potential for the control and management of JD in goat herds (Singh et al., 2007a). Molecular studies by Singh et al. (2014c), reported presence of ‘Indian Bison type’ as major biotype (92 to 95.0%) and rest of the times it was ‘cattle type’. Since affected cows in dairy farm located at Alwar, Rajasthan also suffered with ‘Indian Bison Type’ biotype strain. Therefore, to restore the health and productivity of H/F dairy cow and to salvage dairy farm consisting of high yielding cows from closure, cows were vaccinated using a goat based ‘indigenous vaccine’ after outbreak in October, 2013. This study has no parallel in India.
Clinical symptoms of JD are non-specific and affected cows exhibited progressive weakness with or without diarrhoea in small ruminants. Whereas in native breeds of cows non-treatable continuous diarrhoea has been the main symptom of JD. However, present study report first time loss in body condition and body weights, sharp decline in milk production and progressive weakness without diarrhoea in the dairy farm consisting of high yielding Holstein and Frisian cows, located in Alwar district of Rajasthan. Similar symptoms have been noticed by senior author, while monitoring status of JD in endemically infected goat herds in past 30 years, wherein goats exhibited progressive weight loss both with and or without diarrhoea. Present outbreak of JD was confirmed in the laboratory using multiple diagnostic tests (Singh et al., 2014a). In the present study, first time in India goat based ‘indigenous vaccine’ has successfully salvaged a dairy farm consisting of high yielding H/F cows from eminent closure and economic disaster. Heavy losses incurred by farmer were in term of loss in milk yield, weakness, reduced fertility, deaths, removal of un-productive cows, increased veterinary cost etc. Senior author have already demonstrated ‘Therapeutic potential’ of the goat based ‘indigenous vaccine’ in goat herds and sheep flocks affected with Johne’s disease for long time (Singh et al., 2010; Singh et al., 2013). Similarly, Gudair and Silirium vaccines have been used in Spain to control JD in the livestock population (Bastida et al., 2011).
‘Indigenous Johne’s disease vaccine’ have been found to be effective and had quick response as ‘therapeutic vaccine’ on the basis of improvements shown in different production parameters (high immune response, reduced shedding of MAP, low morbidity and mortality). Present paper report data on monitoring of vaccine response and improvement in the six months period after vaccination, since at the time of vaccination most of cows were in advance clinical stage of JD. Usually affected animals in the farms endemic for JD are in different atages (mostly advance atage) of developing Johne’s disease, therefore vaccine response takes longer time (at least one year) to record improvement in all affected cows. Present study reported significant improvement in most of the health and production parameters of affected dairy cows after one time vaccination against JD using goat based ‘Indigenous Vaccine’ at the face of outbreak. Cows are under continuous monitoring to know how long (after one year post vaccination) this therapeutic effect would be sustained by vaccinated cows. Cows exhibited significant improvements in physical profile and production parameters (improvement in milk yield, gain in body weights and conditions), mortality, morbidity, forced removal etc. since nutritional status of cows was optimum. Affected cows in next parity also remained high milking status after one time vaccination.
Reduction in number of MAP shedders was not statistically significant after 6 months (180 DPV) of vaccination, however, bacterial burden reduced by 46.6% by microscopic examination. Similar findings were reported previously by Hines et al. (2007). Reduction in shedding of herds MAP by cows was also observed following vaccination with live (Jorgensen, 1984) and killed (Kalis et al., 2001) vaccines. HSP70 based therapeutic recombinant vaccine was found effective in reducing bacterial shedding in cattle infected with MAP (Keeble and Walker, 2009). However, few studies indicated contradictory findings where authors did not found evidence of bacterial reduction in feses after vaccination against JD (Wentink et al., 1994; Koets et al., 2006).
Mortality and morbidity were reduced by ‘indigenous vaccine’ after vaccination in last six month period of the vaccination as compared to six month vaccination. Interestingly, in short period after vaccination, considerable reduction was noticed in morbidity due to weakness, diarrhea, and other diseases. That may be possible, due to the fact that vaccine provides cross protection from other bacterial diseases (Syam et al., 2014).
Humoral response proved that antibody titre appear late in infections and was prominent during subclinical and clinical stage. Detection MAP antibodies by ELISA was suggested in the large scale JD control programs in dairy herds since test is quick, cost-effective, and high throughput. Indigenous MAP antibody ELISA kit showed (42.1%) sensitivity and (95.1%) specificity (Singh et al., 2009) was used in the present study. The vaccine used in the present study revealed high ELISA titer indicating sero-conversion of vaccinated cows. Significant difference was observed (P<0.05) in number of ELISA reactors at 60 DPV in vaccinated groups. While no significant difference (P>0.05) was observed at 120 and 180 DPV. All the cattle in vaccinated group became ELISA positive at or after 60 DPV of vaccination. Significantly high (P<0.05) number of cows in vaccinated group retained high titers of anti-MAP antibodies over the experimental periods (Table 2). High sero-conversion rates were observed in cattle of vaccinated group as compared to the control group. Sero-positive animals reported lowered milk production previously by Beaudeau et al. (2007).
The remuneration obtained from increase in production, compensated losses caused by reduction in clinical cases, mortality, morbidity and removal of cows in following six months after vaccination vis a vis six months before vaccination. New plans for the introduction of improved MAP vaccines for cattle (Vordermeier et al., 2011), will also affect the prospects of MAP vaccination in cattle, since the associated DIVA test will be needed to differentiate MAP infected versus MAP vaccinated animals.
The study showed the significant and effective response of goat based indigenous vaccine in saving high yielding H/F dairy cows suffering with outbreak of JD and heavy losses, since biotype of MAP infecting dairy herd and in vaccine used were similar (‘Indian Bison Type’). ‘Indigenous vaccine’ helped to restore the lost productivity and health of affected dairy cow of H/F breed from outbreak of JD and restored confidence of farmer in dairy farming. Therefore, ‘Indigenous vaccine’ can be used to salvage large population of cows, let off by their owners due to zero milk production in the country.
Conflict of Interest
No conflict of interest to declare.
Acknowledgement
Authors are thankful to CSIR, New Delhi for providing the funds and Director, CIRG, Makhdoom for providing the facilities.
References





