Advances in Animal and Veterinary Sciences
Research Article
Clinical Association of Chicken Anaemia Virus with Other Infectious Poultry Diseases in North India and Nepal: Its Pathological Studies, Molecular Epidemiology and RFLP Pattern of PCR Amplified Full Length Viral Genome
Gopal Krishan1, Santosh Kumar Shukla2, Prakash Bhatt3, Maqbool Yaqoob Wani4, Kuldeep Dhama5, Yash Pal Singh Malik6, Rajesh Kumar7*
1Department of Animal Husbandry, Himachal Pradesh, India; 2Department of Veterinary Medicine; 3Veterinary Clinics, 7Department of Veterinary Microbiology, College of Veterinary and Animal Sciences, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Udham Singh Nagar, Uttarakhand - 263 145, India; 4Immunology Section, 5Avian Diseases Section, Division of Pathology, 6Division of Biological Standardization, Indian Veterinary Research Institute (IVRI), Izatnagar (U.P.) – 243 122, India.
Abstract | The aim of the study was to assess the role of chicken anaemia virus (CAV) in precipitating important poultry diseases in commercial poultry flocks and its molecular surveillance in poultry flocks of North India (Uttarakhand and Uttar Pradesh states) and Nepal (Mahendra Nagar). Study was conducted during September-2012 to April-2013 on 13 randomly selected poultry flocks. Necropsy was performed for making tentative diagnosis of other concomitant diseases and samples were collected and epidemiological data was recorded. Flocks were screened for CAV by VP2 gene based PCR followed by PCR-RFLP analysis of viral genome. Haemato-biochemical parameters of flocks were also studied. Out of 185 tissue samples, PCR screening confirmed the presence of CAV genome in 95 samples, while 50 showed other viruses like infectious bursal disease (IBD), Inclusion Body Hepatitis (IBH) and Marek’s Disease (MD). PCR-RFLP analysis of the full genome of different CAV isolates with Hind III, Pst I, Nhe I and Mbo II restriction enzymes showed indistinguishable restriction patterns. A significant decline in erythrocytic and leukocytic cell counts, and increase in the activities of degenerating enzymes was observed in CAV positive flocks. In conclusion, CAV was detected at different poultry farms of India and Nepal and its association with precipitation of other poultry diseases through its marked immunosuppressive potential is evident from findings of present study. Thus, the role of CAV in precipitating other infectious diseases in birds along with molecular epidemiology and immunosuppressive effects needs to be studied in detail so that a comprehensive strategy may be formulated for timely adoption of effective prevention and control measures, subsequently alleviating economic losses in poultry rearing countries.
Keywords | Chicken anaemia virus, Poultry, Immunosuppression, Haemato-biochemical parameters, PCR- RFLP, Molecular epidemiology
Editor | Muhammad Munir (PhD), Avian Viral Diseases Programme, Compton Laboratory, Newbury, Berkshire, RG20 7NN, UK.
Received | May 20, 2015; Revised | June 12, 2015; Accepted | June 13, 2015; Published | June 17, 2015
*Correspondence | Rajesh Kumar, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Udham Singh Nagar, Uttarakhand- 263 145, India; Email: [email protected]
Citation | Krishan G, Shukla SK, Bhatt P, Wani MY, Dhama K, Malik YPS, Kumar R (2015). Clinical association of chicken anaemia virus with other infectious poultry diseases in North India and Nepal: Its pathological studies, molecular epidemiology and RFLP pattern of PCR amplified full length viral genome. Adv. Anim. Vet. Sci. 3(7): 395-405.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.7.395.405
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Krishan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Chicken anaemia virus (CAV), the causative agent of chicken infectious anaemia (CIA), belongs to the Gyrovirus genus of the Circoviridae family (Pringle, 1999; Todd et al., 2000), and is an economically important and emerging pathogen affecting poultry industry worldwide (McNulty, 1991; Jiang et al., 2005; Dhama et al., 2008; Schat, 2009; Kim et al., 2010; Oluwayelu, 2010; Bhatt et al., 2013; Snoeck et al., 2012; Nayabian et al., 2013; Wani et al., 2013). CAV is a non-enveloped virus with a circular, covalently linked, negative-sense, ss DNA genome of about 2,298 to 2,319 nucleotides, and is one of the smallest avian viruses (23-25 nm size). The major transcript from the CAV genome is an unspliced polycistronic mRNA of about 2100 nucleotides encoding VP1 (51.6 kDa), the viral capsid protein; VP2 (24.0 kDa), a scaffold protein; and VP3 or apoptin (13.6 kDa) that is responsible for causing apoptosis in chicken thymocytes and chicken lymphoblastoid cells (Todd et al., 1992; Natesan et al., 2006).
Chicken anaemia virus infection is characterized by lymphoid atrophy, immunosuppression, increased mortality, aplasia of the bone marrow and anaemia, reduced body weight gain, and the development of subcutaneous and intramuscular haemorrhages in chickens (Dhama et al., 2008; Schat, 2009; Pope, 1991; Miller and Schat, 2004). No specific treatment exists for CIA, supplementation with haematinics and immunostimulants can ameliorate anaemic condition and depressed immunity (Bhatt et al., 2013; Latheef et al., 2013). Vaccines including of live-attenuated and killed vaccines are being used but have some limitations, and therefore DNA vaccine and other advanced vaccine options are being explored (McNulty, 1991; Schat, 2003; Dhama et al., 2008, 2014, 2015; Sawant et al., 2015; Zhang et al., 2015). CAV is considered to play an important role in precipitation of number of poultry diseases (Dhama et al., 2008). Experimental co-infection of CAV with Marek’s disease virus (MDV) (Zanella et al., 1999; Miles et al., 2001), dual infection of CAV with infectious bursal disease virus (IBDV) (Yuasa et al., 1980; Rosenberger et al., 1989; Imai et al., 1991; McNeilly et al., 1995), or adenovirus (Toro et al., 2000 and 2001) have resulted in increased morbidity and mortality. CAV infection in poultry has also been associated with poor efficacy of vaccines against Marek’s disease, Newcastle disease and infectious laryngotracheitis (Box et al., 1988; Otaki et al., 1988; Cloud et al., 1992; Liu et al., 2001) Precipitation and/ or increase in mortality due to these diseases by concomitant CAV infection owes to immunosuppression caused by CAV (McNulty, 1991; Dhama et al., 2008; Pope, 1991).
CAV has been reported from poultry flocks of India based on clinical signs, virus isolation, serological and molecular detection studies (Natesan et al., 2003; Dhama et al., 2004; Bhatt et al., 2011; Wani et al., 2013), however, detailed molecular epidemiological studies, immunosuppression, clinical presentation and association of the virus with other infectious pathogens/diseases of poultry is lacking. Applications of molecular tools like PCR along with RE analysis and sequencing have emerged as effective confirmatory tools for diagnosis and studying the molecular epidemiology of poultry pathogens including CAV, which help in designing and adapting appropriate disease prevention and control programme. However, applications of clinical diagnostic methods based on haemato-biochemical parameters help in devising the effective treatment strategies. Present study describes the molecular epidemiology of CAV and its role in precipitating important infectious diseases of poultry in commercial flocks of northern Indian states (Uttarakhand and Uttar Pradesh) and Nepal.
MATERIALS AND METHODS
Sample Collection
Epidemiological data of different infectious diseases from the poultry farms of Uttarakhand state (Udham Singh Nagar, Nainital districts), Uttar Pradesh (Bareilly, Pilibhit and Rampur districts), India and Nepal (Mahendra Nagar district) were recorded during the period of September-2012 to April-2013 (Table 1). Blood samples were collected from affected birds of all the flocks under investigation at the beginning of different disease outbreaks. Five apparently unhealthy birds were selected from each flock, and 2 ml of blood was collected in heparinised vials (10-20 IU/ml) for haemato-biochemical studies and in sterilized test tubes for separation of serum. Post-mortem examination of at least three dead birds from each farm was carried out. Detailed gross post-mortem lesions were recorded and tentative diagnosis was made. Tissue samples from liver, thymus, spleen, bursa of fabricius and bone marrow were collected in sterilized vial using aseptic conditions and kept in air tight containers at -20°C for PCR detection of CAV genome.
PCR Detection of CAV
For the detection of CAV by PCR in clinical samples, whole tissue DNA was extracted from thymus, bursa, spleen, liver and bone marrow using GeneiPureTM Mammalian Genomic DNA Purification Kit (Bangalore Genei) as per manufacturer’s recommendations. The absorbance of the extracted DNA was measured at 260 nm and 280 nm in UV-VIS spectrophotometer. The ratio of OD260 and OD280 was calculated to check the purity of extracted DNA. The DNA samples having ratio of ≥ 1.8 were used for PCR. The amplification of CAV VP2 gene was carried out using sense VP2F: 5‘ATG CAC GGG AAC GGC GGA C3’ and antisense VP2R: 5‘TCA CAC TAT ACG TAC CGG GG3’ primers as described previously (Basaraddi et al., 2013). The PCR products were confirmed by running in l % (w/v) agarose gel in 0.5X TBE buffer with DNA bands stained with ethidium bromide (0.5µg/ml) as per the method of Sambrook et al. (1989), and visualized and documented using Gel documentation system (Alpha Imager).
Restriction Endonuclease (RE) Analysis
Full genome of the virus was amplified by using primers viz., FGF: 5’-GAA TTC CGA GTG GTT ACT ATT CCA TCA-3 and FGR: ‘5’-GAT AGT GCG ATA AAT CTA TTT TCT GCG T-3’. The amplified products were subjected to restriction endonuclease (RE) enzyme digestion using Hind III, Pst I, Nhe I and Mbo II restriction enzymes as per the manufacturer’s protocol, using specific assay conditions and the buffers supplied (Merck Genei). The molecular weights of various fragments of the digested viral DNA were determined using Fortran Software and expressed in base pairs (bp).
Haemato-biochemical Analysis
The various haematological parameters viz., haemoglobin (Hb) content, packed cell volume (PCV), total erythrocyte count (TEC), total leukocyte count (TLC) and differential leukocyte count (DLC) were estimated by standard procedures. For biochemical parameters, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphtase (ALP) and uric acid (UA) were estimated using commercially available reagent kits.
RESULTS
Prevalence of CAV and Co-infections
During the disease investigation in the affected poultry flocks, birds were found suffering from chicken infectious anaemia– gangrenous dermatitis syndrome (CIA-GDS), Marek’s disease, infectious bursal disease and inclusion body hepatitis (Figure 1 and Table 2). In the PCR based screening of a total of 185 tissue samples collected from twelve different flocks, 145 samples (78.38%) were found positive for CAV (95/95), Marke’s disease (15/15), infectious bursal disease (20/20) and inclusion body hepatitis (15/15), while 40 samples tested negative for fowl pox, CRD and gout. The CAV positive flocks showed virus positive PCR amplicons of 651 bp (VP2 gene) and ~2.3 kb (complete genome) (Figure 2). The detailed results of the molecular surveillance of CAV in the affected poultry flocks are presented in Table 3.
Table 1: Details of poultry flocks
|
Sr. No. |
Farm Code/Area |
Code No. |
System of management |
Age of Birds |
Total Flock Strength |
|
1. |
N (Baazpur, UK*) |
N |
Cage |
14 weeks |
6000 |
|
2. |
SP (Baazpur, UK) |
SP |
Cage |
12 weeks |
12000 |
|
3. |
KP (Kashipur, UK) |
KP |
Cage |
23 weeks |
9000 |
|
4. |
NP (Jaffarpur, UK) |
NP |
Cage |
17 weeks |
8000 |
|
5. |
IP (Punjabnagar, UP*) |
IP |
Deep litter |
2 weeks |
1000 |
|
6. |
DP (Ramnagar, UK) |
DP |
Deep litter |
3 weeks |
1200 |
|
7. |
AP (Bilaspur, UP) |
AP |
Deep litter |
3 weeks |
2400 |
|
8. |
ZP (Pilibhit, UP) |
ZP |
Deep litter |
5 weeks |
2000 |
|
9. |
PN (Golapar, UK) |
PN |
Deep litter |
4 weeks |
1200 |
|
10. |
AH (Baazpur, UK) |
AH |
Cage |
16 weeks |
9000 |
|
11. |
PI (Pilibhit, UP) |
PI |
Deep litter |
6 weeks |
1300 |
|
12. |
PF (Mahendra Nagar, Nepal) |
PF |
Deep litter |
2 weeks |
1800 |
|
13. |
MP (Bareilly, UP) |
MP |
Deep litter |
7 weeks |
900 |
* UK = Uttarakhand (India), UP = Uttar Pradesh (India)
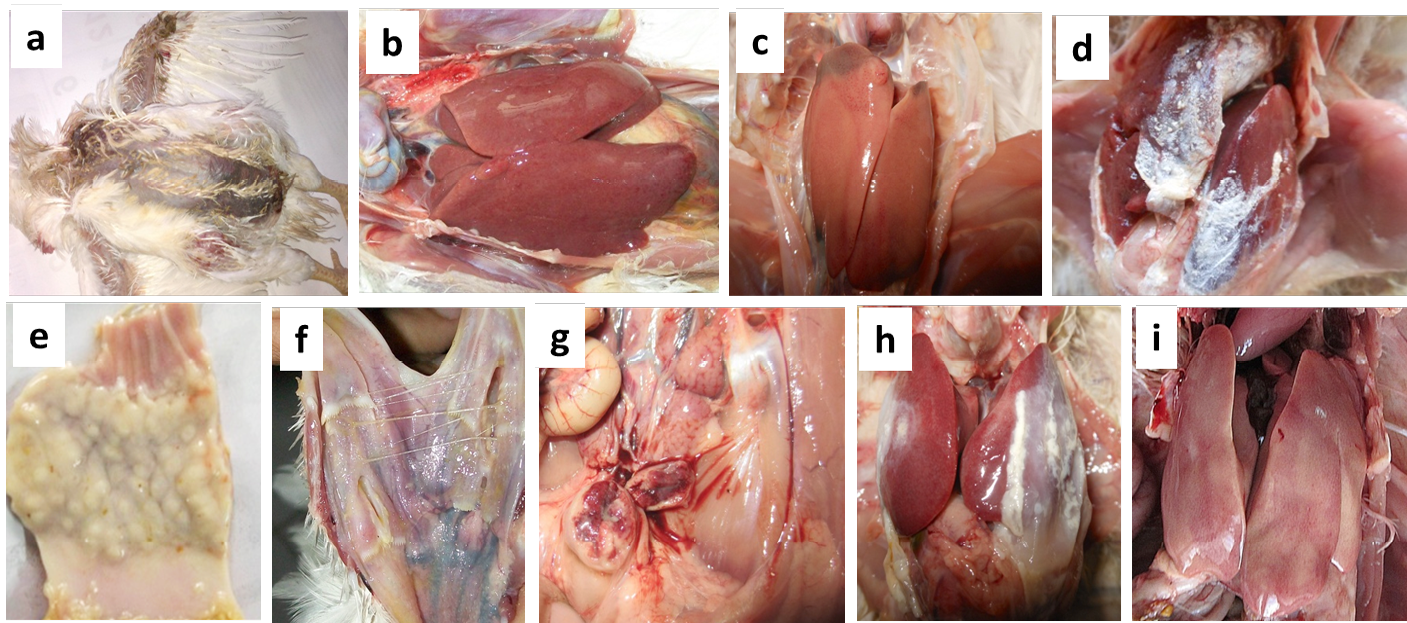
Figure 1: Necropsy findings of the suspected CAV infected poultry birds collected from different farms
(a) Moist haemorrhages on the skin of CIA-GDS affected bird; (b) Nodular appearance of liver in Marek’s disease affected bird; (c) Swollen liver with necrotic foci in CIA-GDS affected bird; (d) Urate deposits in Gout affected bird; (e) Thickened proventriculus in Marek’s disease affected bird; (f) Caseous plug on opening of trachea in Fowl pox affected bird; (g) Haemorrhages on mucosal surface of bursa in IBD affected bird; (h) Pericarditis and perihepatitis in CCRD affected bird and (i) Punctate haemorrhages on the liver of IBH affected bird
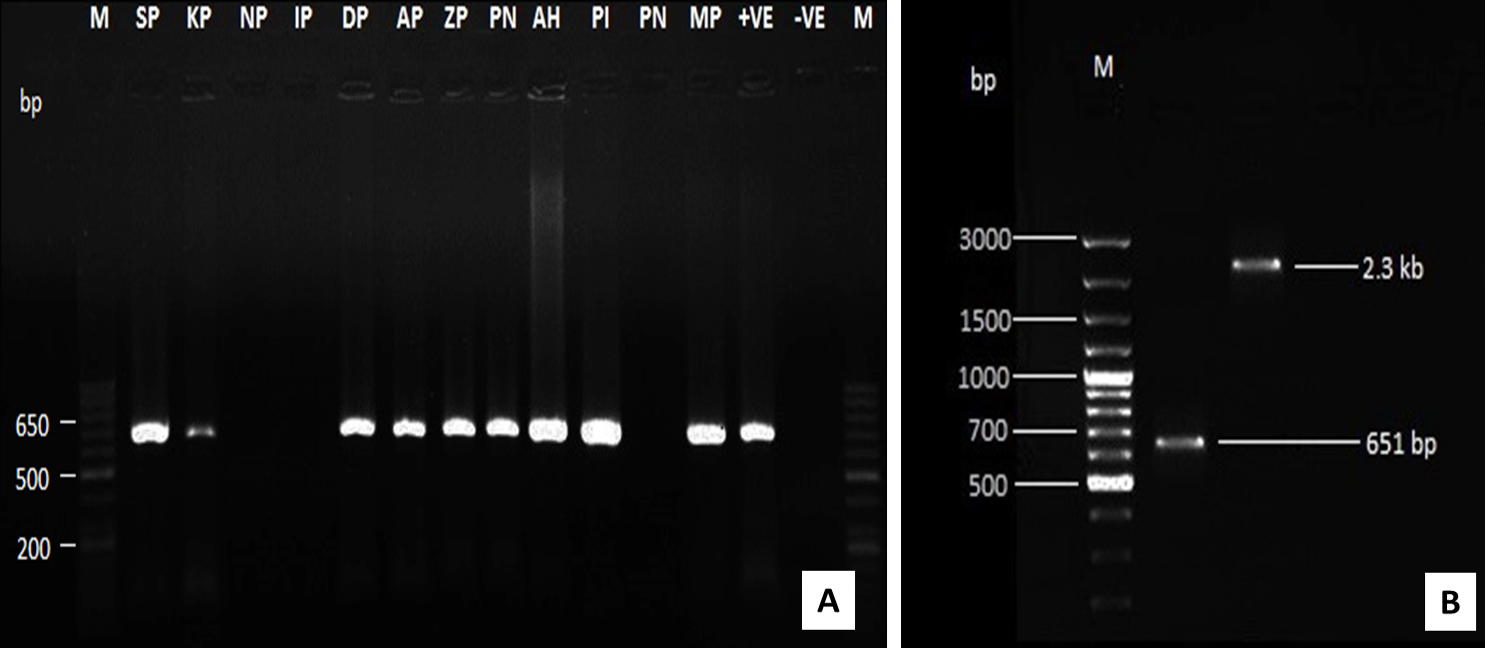
Figure 2: Amplification of CAV-DNA using PCR assay using VP2 specific primer (A) and by using both VP2 and full length genome primers (B)
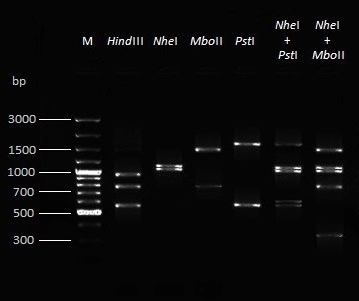
Figure 3: Restriction fragment length polymorphism (RFLP) pattern after RE digestion of amplified full length CAV genome with four different enzymes and their combinations
Restriction Enzyme (RE) Analysis of Full Genome CAV Isolates
RE analysis of full genome amplicon (~2.3 kb) of all CAV isolates with HindIII, PstI, NheI and MboII restriction enzymes revealed identical RFLP patterns (Figure 3, Table 4). All amplicons contained three HindIII sites, one PstI site, one NheI site and one MboII site.
Analysis of Haematological Parameters
Haematological parameters of CAV positive and negative groups revealed a significant (P<0.05) decline in the Hb, PCV, TEC, mean Hb and mean PCV in the CAV positive groups as compared to the control group (Table 5). In comparison to healthy control, a significant (P<0.05) reduction in the mean TEC values of CAV positive flocks was observed except the one farm (KP). In the myeloid lineages also, a significant (P<0.05) decline in the TLC, percent lymphocyte count (PLC) and mean PLC was observed in all the CAV positive birds as compared to the negative group. The mean TLC was found significantly (P<0.05) lower in the all the CAV positive flocks except in one positive flock (MP) which was suspected for IBH based on gross lesions. All the CAV positive flocks showed a significant (P<0.05) increase in the mean percent heterophil count as compared to the negative control group.
Analysis of Biochemical parameters
All serum samples showed significantly (P<0.05) higher activities of AST, ALT and ALP enzymes in all the CAV positive flocks (Table 6). The mean uric acid value was found significantly (P<0.05) higher in the CAV positive flocks except in one CAV positive farm (KP) when compared with the negative group.
Table 2: Necropsy findings and molecular surveillance of suspected twelve poultry flocks for CAV with their respective tentative diagnosis
|
Sr. No. |
Farm Code |
Necropsy findings |
Tentative diagnosis |
Mort ality (%) |
Type of tissue sample |
No. of samples screened for CAV |
||
|
Total |
Positive |
Negative |
||||||
|
1 |
SP |
- Haemorrhagic and necrotic lesions on the wings and breast region - Severe atrophy of thymus and bursa - Bone marrow aplasia with yellowish discolouration - Pale discolouration of liver |
Chicken Infectious Anaemia Gangren Dermatitis Syndrome |
5.72 |
Thymus, Bursa, Bone marrow, Spleen, Liver |
20 |
20 |
- |
|
2 |
KP |
- Discolouration of liver with presence of nodules - Marked thickening of proventriculus |
Marek’s Disease |
2.67 |
-do- |
15 |
15 |
- |
|
3 |
NP |
- Cheesy plug on opening of trachea - Diptheritic membrane with white nodules along mucosal lining of trachea, upper esophagus and nasal cavity |
Fowl Pox |
1.20 |
-do- |
15 |
- |
15 |
|
4 |
IP |
- Chalky white deposition of urate crystals on heart, liver, kidneys and under the skin - Swollen kidneys with urate deposits in ureters giving white chord appearance |
Gout |
3.50 |
-do- |
15 |
- |
15 |
|
5 |
DP |
- Haemorrhagic and necrotic lesions on the wings, breast and thighs - Thymus and bursal atrophy - Bone marrow hypoplasia with presence of pink colour |
Chicken Infectious Anaemia Gangren Dermatitis Syndrome |
11.25 |
-do- |
15 |
15 |
- |
|
6 |
AP |
- Haemorrhages on thigh muscles - Enlarged bursa with presence of haemorrhages on mucosal surface - Swollen kidneys |
Infectious Bursal Disease |
4.00 |
-do- |
20 |
20 |
- |
|
7 |
ZP |
- Haemorrhagic and necrotic lesions on the wings, breast and thighs - Thymus and bursal atrophy - Pink coloured marrow cavity of femur - Slight enlargement of liver with pale discolouration |
Chicken Infectious Anaemia Gangren Dermatitis Syndrome |
4.15 |
-do- |
15 |
15 |
- |
|
8 |
PN |
- Haemorrhagic lesions on wings - Marked atrophy of thymus with mild bursal atrophy - Pinkish discolouration of bone marrow - Discolouration of liver |
Chicken Infectious Anaemia Gangren Dermatitis Syndrome |
3.75 |
-do- |
15 |
15 |
- |
|
9 |
AH |
- Thymus and bursal atrophy - Bone marrow hypoplasia with presence of pink - Enlargement and mottled appearance of liver - Haemorrhagic and necrotic lesions on wings and breast |
Chicken Infectious Anaemia Gangren Dermatitis Syndrome |
6.39 |
-do- |
15 |
15 |
- |
|
10 |
PI |
- Haemorrhagic and necrotic lesions on wings and breast region - Severe atrophy of thymus and moderate bursal atrophy - Bone marrow aplasia with yellowish discolouration - Pale discolouration of liver |
Chicken Infectious Anaemia Gangren Dermatitis Syndrome |
45.69 |
-do- |
15 |
15 |
- |
|
11 |
PF |
- Fibrinous pericarditis and perihepatitis - Presence of caseous exudates in the air sac - Catarrhal inflammation of nasal passage, trachea and bronchi - Cheesy exudate around the nostrils and eyes |
Chronic Complex Respirat Disease |
3.33 |
-do- |
10 |
- |
10 |
|
12 |
MP |
- Swelling of the liver with presence of punctiform haemorrhagic foci throughout the surface - Pale coloured marrow cavity of the femur - Enlarged and pale kidneys - Mild bursal atrophy |
Inclusion Body Hepatitis |
6.22 |
-do- |
15 |
15 |
- |
Table 3: Disease-wise analysis of samples tested by PCR for detection of CAV DNA
|
Sr. No. |
Suspected Disease (Based on gross lesions) |
No. of samples tested |
No. of positive samples |
No. of negative samples |
|
1. |
Chicken Infectious Anaemia – Gangrenous Dermatitis Syndrome |
95 |
95 |
- |
|
2. |
Marek’s Disease |
15 |
15 |
- |
|
3. |
Fowl Pox |
15 |
- |
15 |
|
4. |
Gout |
15 |
- |
15 |
|
5. |
Infectious Bursal Disease |
20 |
20 |
- |
|
6. |
Chronic Complex Respiratory Disease |
10 |
- |
10 |
|
7. |
Inclusion Body Hepatitis |
15 |
15 |
- |
|
Total |
185 |
145 |
40 |
|
Table 4: Digestion products of RE analysis with different enzymes
|
Sr. No. |
Restriction Enzymes |
Digestion products (bp) |
|
1. |
Hind III |
966, 791, 562 |
|
2. |
Pst I |
1743, 576 |
|
3. |
NheI |
1191, 1128 |
|
4. |
Mbo II |
1522, 797 |
|
5. |
Nhe I + Pst I |
1743, 1191, 1128, 615, 576 |
|
6. |
Nhe I + Mbo II |
1522, 1191, 1128, 797, 331 |
DISCUSSION
CAV infection has been incriminated as the cause in several cases where infectious anaemia occurred in chicken flocks of up to 2-4 weeks of age (McNulty, 1991; Dhama et al., 2008; Schat, 2009; Pope, 1991; Rosenberger and Cloud, 1989). Age related resistance occurs in chicks as clinical disease does not occur in chicks above 4 weeks of age (Wani et al., 2014). Co-infections of CAV with other pathogens can overcome the effects of age and maternal antibody related resistance, thus increasing the susceptible age period for CAV and its persistency (Yuasa et al., 1980; Rosenberger and Cloud, 1989; Toro et al., 2000). In the field, CAV infection seems to cause few signs of disease, however, dual infections generally occur which are more serious in terms of economic losses to poultry producers (Dhama et al., 2008; Pope, 1991; Imai et al., 1999). Therefore, it becomes necessary to determine the existence of CAV in association with other pathogens in poultry population of any country. Although, postmortem findings act as first hand tentative diagnostic method for many infections in poultry, the association of CAV in mixed infections and its diagnosis is difficult especially during subclinical infection. Applications of molecular tools have paved a way for the rapid and convenient diagnosis in such mixed infections.
In India, chicken infectious anemia (CIA) has been suspected since 1990’s after the emergence of virulent strains of IBDV and on the basis of clinical symptoms, lesions and its detection by immunoperoxidase test (Venugopalan et al., 1994). Presently, the disease has been confirmed from many states using virus isolation, PCR detection and serological studies (Wani et al., 2013; Natesan et al., 2003; Dhama et al., 2004; Bhatt et al., 2011). The molecular techniques including PCR have been well employed by several workers (Wani et al., 2013; Todd et al., 1966; Dhama et al., 2004; Bhatt et al., 2011; Rozypal et al., 1997; Chowdhury et al., 2002; Miller et al., 2003) for detection of CAV-DNA in clinical samples. However, detailed molecular epidemiological status, immunosuppressive nature and clinical/field interactions of the virus with other infectious pathogens/diseases of poultry have not been performed earlier in the country.
In the present study, molecular surveillance of the CAV, IBD, IBH, fowl pox, gout, CRD and MD in suspected poultry flocks from north Indian states and Mahendra Nagar area of Nepal with the history of different disease outbreak, viz. bacterial, viral, protozoal, etc. was undertaken along with clinical, pathological and haemato-biochemical parameters studies. High prevalence of CAV recorded in present study is in accordance with the earlier reports from India (Wani et al., 2013; Bhatt et al., 2011). Amongst the nine CIA positive flocks, three flocks were suffering with MD, IBD and IBH based on necropsy examination results. Jin et al. (2001) found co-infection of MDV, CAV and REV in some tissue samples of bursa from the chicken flocks suspected to be infected by IBDV according to clinical symptoms and gross lesions by dot hybridization technique in some parts of China. Later, Jiang et al. (2005) also found co-infection of MDV, CAV and REV in tissue samples of 828 chickens from 42 different chicken flocks suspected to be infected by immunosuppressive diseases in 11 provinces of China.
Table 5: Mean values of haemoglobin, PCV, TEC, TLC and DLC in different flocks
|
Sr. No. |
Farm Code |
Haemo globin (Hb) (g/L) |
Packed Cell Volume (PCV) (L/L) |
Total Erythrocyte Count (TEC) (x109/L) |
Total Leukocyte Count (TLC) (x106/L) |
Differential Leukocyte Count (DLC) |
|||
|
Heterophil (%) |
Lymphocyte (%) |
Monocyte (%) |
Eosinophil + Basophil (%) |
||||||
|
1. |
N |
118.33 ±0.24 |
0.32±0.00 |
3.20±0.05 |
21.47±0.81 |
33.67 ±0.58 |
60.00 ±0.33 |
3.67 ±0.33 |
2.67±0.33 |
|
2. |
SP |
66.00 ±0.12* |
0.21±0.01* |
1.92±0.04* |
11.56±1.74* |
53.00 ±1.53* |
41.00 ±1.53* |
3.33 ±0.33 |
2.67±0.33 |
|
3. |
KP |
116.00 ±0.12 |
0.30±0.01* |
3.15±0.14 |
17.73±0.18* |
42.00 ±1.15* |
53.00 ±1.53* |
2.66 ±0.67 |
2.33±0.33 |
|
4. |
NP |
109.00 ±0.25 |
0.32±0.01 |
2.74±0.09* |
19.93±0.18 |
55.67 ±0.88* |
39.33 ±0.88* |
3.00 ±0.58 |
2.00±0.00 |
|
5. |
IP |
114.67 ±0.18 |
0.33±0.00 |
2.99±0.12 |
20.47±1.05 |
56.67 ±0.88* |
37.67 ±1.45* |
3.33 ±0.33 |
2.33±0.33 |
|
6. |
DP |
68.00 ±0.12* |
0.24±0.01* |
2.10±0.10* |
9.53±0.42* |
50.00 ±0.58* |
44.67 ±0.88* |
3.33 ±0.67 |
2.33±0.33 |
|
7. |
AP |
72.67 ±0.07* |
0.27±0.02* |
2.72±0.05* |
9.60±0.38* |
50.33 ±1.20* |
44.00 ±1.15* |
3.33 ±0.67 |
2.67±0.33 |
|
8. |
ZP |
68.00 ±0.12* |
0.22±0.00* |
1.95±0.08* |
10.80±0.81* |
49.00 ±1.00* |
44.00 ±1.15* |
3.67 ±0.33 |
2.67±0.33 |
|
9. |
PN |
68.00 ±0.12* |
0.24±0.01* |
2.09±0.09* |
9.73±0.48* |
50.67 ±0.88* |
44.67 ±1.45* |
3.00 ±0.58 |
1.67±0.33 |
|
10. |
AH |
68.67 ±0.18* |
0.23±0.00* |
2.05±0.04* |
9.70±0.46* |
49.67 ±0.67* |
43.67 ±0.88* |
4.00 ±0.58 |
3.00±0.58 |
|
11. |
PI |
65.33 ±0.37* |
0.19±0.01* |
1.69±0.22* |
9.87±0.35* |
49.00 ±1.73* |
43.67 ±2.40* |
4.00 ±0.58 |
2.67±0.33 |
|
12. |
PF |
114.00 ±0.23 |
0.32±0.00 |
2.89±0.15 |
25.67±0.70* |
58.00 ±0.57* |
34.33 ±1.20* |
5.33 ±0.33* |
2.33±0.33 |
|
13. |
MP |
78.00 ±0.23* |
0.26±0.01* |
2.81±0.10* |
22.80±1.36 |
38.33 ±1.20* |
53.00 ±1.00* |
5.67 ±0.33* |
2.67±0.33 |
* Significantly (P<0.05) different
Table 6: Mean value of AST, ALT, ALP, Uric Acid in different flocks
|
Sr. No. |
Farm Code |
AST (IU/L) |
ALT (IU/L) |
ALP (IU/L) |
Uric acid (UA) (mg/dl) |
|
1. |
N |
174.83±0.66 |
34.00±0.31 |
485.50±1.55 |
5.53±0.05 |
|
2. |
SP |
322.33±3.60* |
70.97±0.84* |
586.13±6.23* |
7.00±0.11* |
|
3. |
KP |
256.83±1.55* |
59.91±0.78* |
558.93±1.08* |
5.90±0.17 |
|
4. |
NP |
178.30±2.16 |
34.54±0.83 |
488.13±1.30 |
5.70±0.04 |
|
5. |
IP |
279.67±2.03* |
66.90±0.85* |
572.80±1.64* |
12.27±0.99* |
|
6. |
DP |
263.43±2.75* |
64.23±0.35* |
567.87±3.46* |
6.76±0.44* |
|
7. |
AP |
241.03±4.36* |
54.80±0.97* |
537.10±3.40* |
11.47±0.57* |
|
8. |
ZP |
254.20±1.97* |
57.23±1.28* |
558.10±2.64* |
6.83±0.08* |
|
9. |
PN |
251.13±1.50* |
56.13±1.86* |
552.47±4.22* |
6.73±0.04* |
|
10. |
AH |
265.07±1.68* |
64.23±0.32* |
572.27±2.16* |
6.96±0.09* |
|
11. |
PI |
284.03±6.85* |
54.73±1.36* |
571.43±1.76* |
6.96±0.09* |
|
12. |
PF |
243.90±2.41* |
70.20±0.54* |
542.57±1.81* |
5.82±0.04 |
|
13. |
MP |
324.33±3.60* |
74.53±0.54* |
603.37±2.59* |
7.14±0.08* |
* Mean values (±SE) are significantly different (P<0.05)
In the present investigation, all the PCR amplified full genome DNA amplicons from CAV positive flocks showed identical RFLP pattern with restriction enzymes (Hind III, Pst I, Nhe I and Mbo II), signifying ciruclation of single serotype of the virus. Previous reports have shown pathogenic variations among the different isolates (Kim et al., 2010; Natesan et al., 2003; Yuasa et al., 1986; McNulty et al., 1990; Adair, 2000; Connor et al., 1991; Davidson et al., 2004; Oluwayelu et al., 2005). A study from the USA reported an antigenically different CAV isolate (Spackman et al., 2002), which could be a prototype virus of serotype 2. It has been highlighted that due to recombination mechanisms the new genetic variants are arising (Eltahir et al., 2011). Recently, genetically similar variants of gyroviruses have been detected in the human populations, alarming their importance in human health (Biagini et al., 2013; Smuts, 2014). Todd et al. (1992) assigned 14 CAV isolates of seven countries in to seven groups by RE analysis of the PCR amplified DNA with Hae III, Hinf I and Hpa II enzymes. Noteborn et al. (1992) used the enzymes, viz., EcoRI, AccI, BglII, HindIII, SstI, BamHI, XbaI and noticed some interesting minor differences by AccI, HindIII and EcoR I enzymes in spite of the overall high degree of similarity. Similarly, Imai et al. (1998) analyzed 14 CAV amplified DNA products to Bgl II, Hind III, Pst I and Sac I which showed the amplified region as highly conserved among all the isolates . However, in one of our study (Dhama et al., 2004) we found some variations among the six Indian CAV isolates on the basis of RE analysis of PCR amplified products. Similarly, Natesan et al. (2003) reported differences among Indian isolates of CAV by using three restriction enzymes (Hha I, Dde I and Hae III). The previous reports are based on RE analysis of partial CAV genome segments and the novelty in RE digestion in this present study is that PCR products covering the whole viral genome were employed rather than any CAV gene specific smaller region PCR products, and such studies are lesser revealing RFLP pattern of the whole genome sequence, thus providing a useful molecular data regarding CAV RE analysis and comparison with other isolates of worldwide origin. Further studies including digestion with other specific restriction enzymes, sequence analysis and phylogenetic analysis of CAV genome are recommended for the differentiation and molecular characterization of all the CAV isolates.
During acute phases of the CIA especially in young chicks, CAV causes destructive effects on erythroid and myeloid tissues of bone marrow leading to suppression of differentiation and proliferation of haemopoietic precursor cells. This drastically affects erythropoiesis and myelopoiesis, leading to anaemia and (McNulty, 1991; Dhama et al., 2008). In the present study, a significant (P<0.05) decline in the Hb, PCV and TEC was observed in the CAV positive groups. In the myleiod lineages also, a significant (P<0.05) decline in the TLC and percent lymphocyte count was observed in all CAV positive birds as compared to the selected healthy group. Such haematological studies could be useful in determining the haematological effects of CAV in co-infections with other pathogens, for which purpose explorative studies are suggested.
It is well documented that AST, ALT and ALP are found in the liver and ALP is found in the kidney also (Kaneko et al., 1997). CAV has a wide spread distribution in the body resulting in damage and focal necrosis of liver, kidney and spleen. Haemorrhages in the proventricular mucosa and subcutaneous tissues and muscular haemorrhages within wing tips are sometimes associated with severe anaemia (Dhama et al., 2008; Yuasa et al., 1986; Engstrom et al., 1988). The present study demonstrated significant (P<0.05) elevation in the activities of ALT, AST and ALP enzymes, and uric acid of CAV positive birds, which can be associated with the damage to the organs like liver, kidneys and muscles of CAV affected chicks. Elaborative studies in this direction could make understand the pathology and pathogenesis of the CIA more clearly.
CAV has been found to be associated with both clinical and sub-clinical infections and have substantial effects particularly on commercial broiler performance. Economic losses stem from increased mortality, condemnations, the cost of antibiotics used to control secondary bacterial infections due to immunosuppression, vaccine failures and poor production performance (McNulty, 1991; Dhama et al., 2008; McIlroy et al., 1992). In the present study, all the CAV positive flocks showed significantly lower value of ND HI titres as compared to the normal healthy flocks. This outcome suggested that CAV is responsible for lower immune response against ND vaccination. The destructive effect of CAV due to suppression of the population of both helper (CD4+) and cytotoxic (CD8+) T-Lymphocytes in the thymus as suggested by Hu et al. (1993) and Adair (2000) could be the reason for poor but protective immune response in CAV inoculated chicks. The low antibody titer in CAV infected birds and marked depression of humoral immune response has also been reported by other (Bhatt et al., 2013; Cloud et al., 1992; Liu et al., 2001).
Overall, findings of the present study highlight the high prevalence of CAV in Indian poultry flocks with confirmation of its immunosuppressive role with other pathogens in field conditions. Furthermore, the active presence of CIA viral DNA in the tissue samples of diseased birds along with clinico-pathological and haemato-biochemical findings in the disease outbreaks investigated confirmed the involvement of CAV and its association with precipitation of other poultry diseases through its marked immunosuppressive potential. Though, this appears to be the first report from India indicating association of CAV in precipitating other secondary pathogens/diseases in commercial poultry flocks, additional studies are required to find out the subclinical infection of CAV in commercial poultry flocks by suitable diagnostic assays and its association with other immunosuppressive and opportunistic pathogens in causing significant economic losses to poultry population. The usage of full length viral genome based PCR-RFLP for studying molecular epidemiology of this emerging and economically important virus is the most significant finding of the present study. The recent reports regarding CAV related variants in human population and its potent role in precipitating other infectious diseases in birds further warrants in depth molecular epidemiological studies, antigenic and genomic characterization of viral isolates, so that an appropriate and effective strategy could be designed and adopted for the prevention and control of this important avian pathogen, and alleviate economic losses being suffered in poultry population.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
ACKNOWLEDGEMENTS
The authors are thankful to the Director, Experiment Station, Dean, College of Veterinary and Animal Sciences, Dean, Post Graduate Studies, G.B. Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, and Director, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh for providing necessary facilities to carry out this research work.
REFERENCES






