Advances in Animal and Veterinary Sciences
Effects of Nasal Obstruction on Hormonal and Immune Functions in Wistar Rats
Asma Dorbani*, Abdelmadjid Bairi, Abdelkarim Tahraoui
Applied Neuroendocrinology Laboratory, Department of Biology, Faculty of Science, University Badji Mokhtar BP 12 23000, Annaba, Algeria.
Abstract | Olfaction occurs in many areas such as reproduction, diet, social behavior, or the detection of predators. In order to know if the absence of olfaction could disrupt mammalian endocrine and immune functions, bilateral nasal obstruction (NO) was induced in young rats of the Wistar strain at the 8th postnatal day. Its consequences were examined 24 hours after the treatment (D9), at the end of the obstruction period (D15) and six days after the reopening of the nostrils (D21). Samples of the biological extracts were taken and our results show that the NO causes a stress which would disturb the development of the individual. At the endocrine level, the NO causes hormonal modifications which are generally more marked in the females (increase in the rate of ACTH). At the level of the immune system, NO leads to a decrease in the number of circulating leukocytes and lymphocytes. For the rat, nasal obstruction is therefore a multifactorial stressful situation.
Keywords | Olfactory deprivation, Anxiety, Lymphocytes, ACTH, Wistar.
Received | November 04, 2018; Accepted | February 18, 2019; Published | April 01, 2019
*Correspondence | Asma Dorbani, Applied Neuroendocrinology Laboratory, Department of Biology, Faculty of Science, University Badji Mokhtar BP 12 23000, Annaba, Algeria; Email: dorbaniasma@ymail.com
Citation | Dorbani A, Bairi A, Tahraoui A (2019). Effects of nasal obstruction on hormonal and immune functions in wistar rats. Adv. Anim. Vet. Sci. 7(6): 447-451
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.6.447.451
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Dorbani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The olfactory system is a sensory system specialized in the detection and processing of volatile chemical information, the odorant molecules.
Unlike vision, it is a relatively plastic sensory system. An unknown molecule can still be detected, even if the olfactory sensation is weak at the beginning, the olfactory system will develop later receivers to detect it more effectively (Duchamp-Viret, 2012).
Nasal obstruction causes a disturbance of the airflow passing through the nasal cavity. It is generally due to a decrease in the size of the nasal die of mechanical or functional origin. Although total nasal occlusion is a rare phenomenon, disruption of airflow in the nasal cavity is a common problem and its repercussion is usually underestimated (Vig, 1998). When the nasal cavities are obstructed, the central nervous system sends the motor effector structures motor commands to release a replacement airway. We then observe the establishment of an oral replacement to maintain the ventilatory function. The newly established oral breathing can be divided into two components: an absence of nasal breathing and a chronic opening of the mouth (Schlenker et al., 2000).
This transition is likely to disrupt all nasal functions and have many consequences locally and regionally or globally.
During the postnatal period, bilateral nasal obstruction may result in:
• Respiratory distress by altering the conditioning of the inspired air.
• Partial social deprivation by disrupting congeneric orientation.
• Nutritional disturbances by disrupting udder orientation (breast milk is the only water source for the newborn).
• Thermal imbalance by disrupting orientation to the mother and nest (in nesting species only).
These different factors are known for their ability to stimulate the neuroendocrine response to stress. Bilateral nasal obstruction could therefore be a stressful multi-factorial situation. In the event that it actually results in the establishment of a stress response, the nasal obstruction would represent a chronic stressful situation, or a relatively moderate disturbance lasting for several days (Pacak and Palkovits, 2001).
As part of this study, all observations were focused on the consequences of nasal obstruction on endocrine and immune function. Some blood parameters were measures CBC, glucose and adrenocorticotropic hormone (ACTH). We will see that by disturbing the breathing and the olfaction, the nasal obstruction could constitute a stressful multifactorial situation likely to disturb the development of certain peripheral physiological systems.
Materials and Methods
Biological Material and Animal Care
In the present study, we used wistar rats (Rattus rattus). Ten mixed sex litters were born in the laboratory of the university. All animals were housed in standard cages on a constant 12-h light/12-h dark cycle with controlled temperature and humidity and were given access to food and water ad libitum.
Nasal Obstruction Procedure and Experimental Groups
At the age of 8 days, the litters were divided into three experimental groups. Untreated group (UT) was defined by the complete absence of manipulation (n=10). Sham group (SH) (n=10) and animals with nasal obstruction (NO) (n=16) were first anesthetized for a short period.
They were placed for few seconds in a glass bell containing a cotton wisp impregnated with ether. Once the litters were anesthetized, a bilateral nasal obstruction was performed on NO animals as previously described by Meisami (1976). The selected method consisted in the cauterization of the external nostrils, which is the most common and simple procedure allowing reversible nasal obstruction in neonates. The tissue surrounding the external nostrils was burned by placing a surgical cauterizing instrument on the nostrils, consequently occluding the orifice of the nostrils. This procedure induced a complete nasal obstruction between PND 8 and PND 14 with 100% of the nostrils reopened at PND 15. In sham group, the nostrils were not sealed but the tissue above them was burned by placing the cauterizing instrument about 1–2 mm above each nostril. After the cauterization, the burn was washed with chlortetracycline (Aureomycine Evans 3%) to prevent a possible infection. SH and NO pups were kept warm (371°C) for 1 h and they were then returned to the mothers.
Biological Samples
After these different operations, the rats are sacrificed on day 9, others on day 15 and those remaining on day 21. Different biological extracts are taken from each individual: The blood samples are taken in EDTA tubes to analyze CBC (the cell blood count), the blood glucose is measured immediately with a glucometer. The blood taken with heparin is centrifuged at 1480 rpm for 10 minutes to obtain the serum. This was stored at -20 ° C until steroid hormonal (ACTH) assays were performed. The comparison will be made for all the parameters measured versus the sham and untreated groups.
Statistical Analysis
Data in the text and figure legends are means ± standard errors for the indicated number (n) of animals. Statistical comparisons were made by analysis of variance (ANOVA) and differences were considered to be significant at P <0.05. (Tukey–Kramer test, P>0.05).
Results
Glucose
In NO animals, blood glucose levels decreased significantly 24 hours after nostril obtruction and reached (0.41 ± 0.098) in males and (0.34 ± 0.055) in females. This drop in blood sugar is highly significant (p <0.0001 vs sham and untreated group). In females, hypoglycemia persisted until day 15 (0.49 ± 0.14) and was very significant (p = 0.012). While in males, there is no significant difference. At D21, blood glucose levels are comparable to those of sham and untreated rats, whether in males (0.92 ± 0.04) or in females (1.13 ± 0.034) (Figure 1).
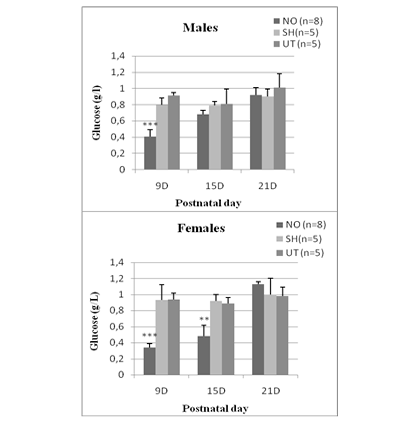
Figure 1: Effect of early nasal obstruction on blood glucose levels (g/L) in NO, sham and untreated rats at 9, 15 and 21 postnatal day.
ACTH (Adrenocorticotrophic Hormone)
Regarding the ACTH plasma concentration, a significant difference between the experimental groups is detected at J9 (F = 23.60, p <0.0001), J15 (F = 27.41, p <0.0001) and J21 (F = 7.96, p = 0.02). Regardless of age, sham and untreated individuals have comparable ACTH concentrations in both females and males (0.36 <p <0.87). On the other hand, animals exposed to nasal obstruction show a significant increase in plasma ACTH. In females, this elevation is observed 24 hours after the treatment (day 9: p <0.0001 vs sham and untreated group), at the end of the nasal obstruction period (J15: p <0.0001 vs sham and untreated) and is maintained until the 21st postnatal day, it reached a high value (2000± 201.4) (pg/ml) compared to untreated (1300±69,4) (pg/ml) and sham groups (1152,1±98,5) (pg/ml) (p <0.0001 vs sham and untreated). In males, the increase was significant on day 9 (p = 0.0003 vs sham group, p = 0.0001 vs untreated group) and on day 15 (p = 0.0004 vs sham , p = 0.0002 vs untreated) but not at J21 (p = 0.19 vs sham, p = 0.14 vs untreated) (Figure 2).
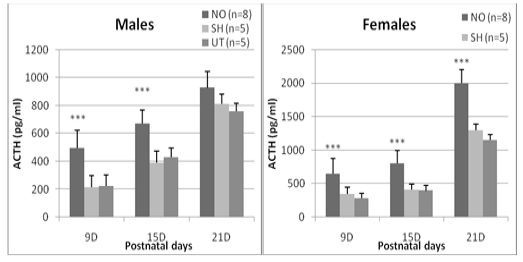
Figure 2: Effect of early nasal obstruction on adrenocorticotropic hormone (ACTH) in experimental groups at 9, 15 and 21 days of age. P: significance level; NS: not significant (P > 0.05).
Cell blood Count (CBC)
The effect of nasal obstruction on hematological parameters in rats is presented in Tables 1 and 2. The results indicate that on some parameters measured at different periods of the experiment (9D, 15D and 21D), only two have varied significantly. In males, very significant changes were observed at the level of lymphocytes on day 9 (74.7 ± 5.22) versus (65.4 ± 5.21) for SH and (62.3 ± 1.21) for UT group, at day 15 (86 ± 2.22) against (71.12 ± 5.01) for SH and (73.51 ± 0.8) for UT group at day 21 (84.2 ± 2.97) for (75.1 ± 2.14) for SH and (69.4 ± 1.55) for UT. Mean monocyte cell concentrations were significant only at D9 (2.8 ± 0.19) versus (6.55 ± 0.55) for SH and (8.15 ± 0.02) for UT group.
Hematological parameters values (Table 2) in rats indicate that in females, nasal obstruction significantly altered white blood cells at D15 (6.82 ± 0.01) against (12.3 ± 2.14) for the SH lot and (13.54 ± 5.1) for the UT lot. This change is maintained until D21 (5.69 ± 0.12) against (9.97 ± 1.45) for the SH group (12.54 ± 4.2) for the UT group. The hematological parameters measured showed highly significant changes in lymphocyte levels, a rate of (43.3 ± 5.01) was recorded on day 9 for the NO group, (35.84 ± 2.7) for the control group and (37.85 ± 1.5) for the sham group, on day 15 the lymphocyte count of NO rats is (61.7 ± 8.54), (49.8 ± 11.21) in SH and UT rats (51,21 ± 9,2). At D21, there were variations for NO of (76.7 ± 2.87) versus (45.5 ± 5.1) for sham and (40.2 ± 7.5) for untreated group.
Discussion
Sensory signals play a key role in establishing and maintaining the two-way relationship between mother and offspring. From a young’s point of view, olfaction is essential for the recognition of the mother and the udders (Blass et Teicher, 1980), nest (Brown, 1982), related or familiar partners (Kristensen et al., 2001).
In Wistar rats exposed to nasal obstruction, weight loss is associated with hypoglycaemia that persists until reopening of the nostrils. Our study also demonstrates a significant increase in the ACTH level which is maintained even at D21 in females. The variations of the external environment are sorted and “felt” essentially by the limbic system which sends this information to the hypothalamus via numerous nerve connections (Gray et al., 1989).
Acute stress increases the amplitude and timing of CRF and AVP pulsations to the pituitary gland. The resulting ACTH peak then induces the synthesis of glucocorticoids by the adrenal glands. Depending on the nature, intensity and duration of the stressor, other factors such as angiotensin II or cytokines may potentiate the secretory action of the various interveners of the corticotropic axis (Chrousos, 1995). The negative feedback control exerted by the glucocorticoids on the different stages of the corticotropic axis then allows the extinction of the peak of secretion involved in response to the stressor.
Our results show a decrease in the level of lymphocytes in the blood, which lasts until the 21st postnatal day. Circulating cells may also be affected by undernutrition. In the cat, seven days of diet cause a drop in the number of leukocytes and circulating lymphocytes (Freitag et al., 2000). These effects are not completely removed after seven days of replenishment. In adult rodents, nutritional deprivation leads to atrophy of the spleen, a reduction in the number of splenocytes and a decrease in their proliferative response (Howard et al., 1999; Cunha et al., 2003).
Indeed, our results show that a nasal obstruction performed from 8th to 15th postnatal day has consequences that last
Table 1: Evaluation of hematological parameters of experimental rats males at 9D, 15D and 21D.
| Postnatal day |
9D |
15D |
21D |
p |
|||||||
|
Group NFS |
NO (n=8) |
SH (n=5) |
UT (n=5) |
NO (n=8) |
SH (n=5) |
UT (n=5) |
NO (n=8) |
SH (n=5) |
UT (n=5) |
||
| WG (10^3/ul) |
6,37± 0,37 |
7,1± 0,25 |
6,95± 1,28 |
7,37± 0,67 |
8,4± 0,05 |
7,56± 0,2 |
8,79± 1,02 |
9 ,45± 2,45 |
7,48± 0,9 |
0,32 ns |
|
| RG(10^6/ul) |
8,94± 0,95 |
9,71± 1,05 |
8,29± 1,87 |
11,24± 0,7 |
10,8± 1,47 |
8 ,32± 0,09 |
10,29± 0,3 |
11,54± 1,8 |
9,70± 0,11 |
0,46 ns |
|
| NEYT (%) |
20,1± 2,53 |
18,1± 1,44 |
17,25± 2,3 |
26± 1,54 |
24,54± 5,02 |
25,32± 3,1 |
36,3± 3,25 |
34 ,47± 2,1 |
32,3± 4,97 |
0,34ns | |
| LYMPH (%) |
74,7± 5,22 |
65,4± 5,21 |
62,3± 1,21 |
86± 2,22 |
71,12± 5,01 |
73,51± 0,8 |
84,2± 2,97 |
75,1± 2,14 |
69,4± 1,55 |
0,017** |
|
| MONO (%) |
2,8± 0,19 |
6,55± 0,55 |
8,15± 0,02 |
6,5± 0,14 |
7,78± 0,51 |
8,14± 1,12 |
8,1± 0,51 |
6,97± 0,09 |
7,1± 1,34 |
0,041 * |
|
| EO (%) |
2,4± 0,33 |
2,14± 0,5 |
1,87± 1,02 |
1,5± 0,21 |
1,69± 0,45 |
2,01± 0,08 |
1,3± 0,44 |
1,8± 0,05 |
1,2± 0,14 |
0,8ns | |
Table 2: Evaluation of hematological parameters of experimental rats females at 9D, 15D and 21D.
| Postnatal day |
9D |
15D |
21D |
p |
||||||
|
Group NFS |
NO (n=8) |
SH (n=5) |
UT (n=5) |
NO (n=8) |
SH (n=5) |
UT (n=5) |
NO (n=8) |
SH (n=5) |
UT (n=5) |
|
| WG (10^3/ul) |
9,60± 0,05 |
11,54± 2,3 |
10,54± 0,2 |
6,82± 0,01 |
12,3± 2,14 |
13,54± 5,1 |
5,69± 0,12 |
9,97± 1,45 |
12,54± 4,2 |
0,032** |
| RG (10^6/ul) |
9,74± 1,21 |
8,78± 3,25 |
10,54± 0,9 |
8,88± 1,21 |
9,32± 2,01 |
9,45± 3,12 |
10,44± 0,4 |
9,47± 0,19 |
10,61± 1,7 |
0,445 ns |
| NEYT (%) |
27,7± 0,21 |
23,54± 2,1 |
26,21± 5,8 |
52,8± 3,77 |
52,14± 9,54 |
49,56± 8,1 |
44,6± 5,41 |
55,98± 2,2 |
60,7± 6,14 |
0,13 ns |
| LYMPH (%) |
43,3± 5,01 |
35,84± 2,7 |
37,85± 1,5 |
61,7± 8,54 |
49,8± 11,21 |
51,21± 9,2 |
76,7± 2,87 |
45,5± 5,1 |
40,2± 7,5 |
0,009 *** |
| MONO (%) |
4,3± 0,22 |
5,1± 0,27 |
6,7± 5,12 |
7,1± 4,14 |
6,22± 0,47 |
4,98± 0,25 |
7,6± 0,06 |
6,12± 4,11 |
4,8± 2,14 |
0,2ns |
| EO (%) |
0,1± 0,04 |
1,14± 0,17 |
0,9± 0,01 |
1,2± 1,27 |
1,5± 0,45 |
1,37± 0,58 |
0,9± 0,19 |
1,44± 0,48 |
1,2± 0,33 |
0,6ns |
at least up to 21 days. Early nasal obstruction is therefore likely to have a more or less long-term impact. Early experiences, including stressful experiences, are known to have consequences that persist into adulthood. Thus, postnatal maternal separation and undernutrition can disrupt the neuroendocrine functions of the adult by modulating, for example, the activity of the corticotropic axis, the somatotropic axis or the secretion of insulin (Harel et Tannenbaum, 1995; Anisman et al., 1998; Houdijk et al., 2003). Interestingly, it appears that the immune damage observed following exposure to conventional stressors (electric shock, physical stress, experimentation by the experimenter, forced swimming or running, etc.) is rapidly reversible after stopping the stressful situation (Shurin et al., 1994; Dhabhar et al., 1995). In contrast, exposure to social stressors (maternal separation in the young or social conflict in the adult male) appears to have long-term immune consequences such as a persistent reduction in T-cell proliferation (Stefanski et Engler, 1999).
Conclusion
The experimental work outlined in this article shows that early nasal obturation disrupts the individual’s balance. This procedure nevertheless constitutes a multi-factorial situation and it appears difficult to understand the interactions and the relative importance of the various factors. These can act synergistically or antagonistically, systemically or specifically to adapt the individual to the new constraints of the environment.
Conflict of Interest
There is no conflict of interest.
Authors Contribution
All authors contributed equally.
References






