Advances in Animal and Veterinary Sciences
Research Article
Screening of Human Population for Type 1 Diabetes and Mycobacterium avium Subspecies paratuberculosis from Chattarpur District of Madhya Pradesh
Shoor Vir Singh1, Narottam Das Agrawal1, Naveen Kumar1, Saurabh Gupta1, Kundan Kumar Chaubey1, Krishan Dutta Rawat1, Ruchi Tiwari2, Jagdip Singh Sohal3, Tarun Kumar Sachan1, Vibhuti Sharma1, Kuldeep Dhama4
1Animal Health Division, Central Institute for Research on Goats, Makhdoom, Farah-281122, Mathura, Uttar Pradesh; 2Department of Microbiology, Veterinary University, Mathura -281 001; 3Amity Institute of Microbial Technology, Amity University Rajasthan, Kant Kalwar, NH 11C Delhi-Jaipur Highway-303 002, Jaipur, Rajasthan; 4Division of Veterinary Pathology, Indian Veterinary Research Institute, Izatnagar-243122, Uttar Pradesh, India.
Abstract | Mycobacterium avium subspecies paratuberculosis, the cause of granulomatous enteritis in ruminants, has also been associated with Inflammatory Bowel disease or Crohn’s disease in human population. Present study aimed to investigate bio-presence of MAP in suspected and confirmed cases of Type 1 Diabetes in clinical samples (blood and serum) of human beings from Chattarpur district of Madhya Pradesh. Screening of 88 serum samples for the presence of MAP using ‘Indigenous ELISA’, 34 (38.6%) were positive for MAP infection. Whereas of the 71 blood samples screened, 28 (39.4%) were positive in IS900 PCR. Screening of 19 serum and 16 blood samples from 20 confirmed patients of ‘Type 1 diabetes’, 31.5 and 43.7% were positive by ‘Indigenous ELISA’ and IS900 PCR, respectively. Comparison of Indigenous ELISA and IS900 PCR revealed fair agreement between the two tests. Bio-typing of DNA of 8 positive blood samples by IS1311 PCR-RE revealed presence of ‘Indian Bison type’ biotype. Presence of MAP bacterimia in the patients suffering from Type 1 Diabetes added to the existing knowledge that MAP may provide foundation for establishing diabetes in human subjects. This is the first report of presence of MAP in human Type 1 Diabetes patients from India. ‘Indian Bison Type’ biotype in domestic livestock suggests a link and interspecies transmission through food chain.
Keywords | Mycobacterium avium subspecies paratuberculosis, Type 1 Diabetes, Indigenous ELISA, IS900 PCR
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 04, 2014; Revised | November 22, 2014; Accepted | November 23, 2014; Published | November 28, 2014
*Correspondence | Shoor Vir Singh, Central Institute for Research on Goats, Makhdoom, Uttar Pradesh, India; Email: shoorvir_singh@rediffmail.com
Citation | Singh SV, Agrawal ND, Kumar N, Gupta S, Chaubey KK, Rawat KD, Tiwari R, Sohal JS, Sachan TK, Sharma S, Dhama K (2014). Screening of human population for Type 1 Diabetes and Mycobacterium avium subspecies paratuberculosis from Chattarpur district of Madhya Pradesh. Adv. Anim. Vet. Sci. 2(11): 612-619.
DOI | http://dx.doi.org/10.14737/journal.aavs/2014/2.11.612.619
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2014 Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Mycobacterium avium subspecies paratuberculosis (MAP) infects wide variety of animals and is endemic in the domestic livestock population of the country (Singh et al., 2010a). MAP has also been associated with Inflammatory Bowel disease (Crohn’s disease) in human beings (Singh et al., 2008). Live cultivable MAP bacilli have been reported from milk based food items (ice creams, cheese, condensed milk, milk powder etc) world-wide (Grant et al., 2001; Ayele et al., 2005; Shankar et al., 2010). In India, raw milk serves as base of many Ayurvedic medicines. Recently role of MAP has been reported in different kinds of human diseases such as Type-1 Diabetes (Sechi et al., 2008), autoimmune thyroiditis (D’Amore et al., 2010), multiple sclerosis (Cossu et al., 2013), autism (Dow, 2011), sarcoidosis (EL-Zaatari et al., 1996) and autoimmune arthritis (Moudgil et al., 1997). Recent years witnessed a surge in cases of Type-1 Diabetes particularly the ‘Type 1 diabetes mellitus’, a chronic disease in which insulin producing beta cells of the pancreas are selectively destroyed by T lymphocyte infiltration, in human beings. Disease is the second most common chronic disease during childhood. Annual incidence of the Type 1 Diabetes (per 100,000) is consistently increasing and the highest being in European countries (32.5), followed by Non-Europeans (14.4), Maori (13.9), Pacific Islanders (15.4) and others (13.5) (Derraik et al., 2012). In Northern India, occurrence of Type 1 Diabetes was found to be 10.20 per 100,000 population with a higher prevalence in urban (26.6/100,000) as compared to rural areas (4.27/100,000). In the age group of 5-16 years, prevalence is 22.22/100,000, while in 0-5 years age group prevalence is 3.82/100,000 (Kalra et al., 2010).
Susceptibility to Type 1 Diabetes is inherited, but mode of inheritance is not clearly understood. Several loci, primarily HLA and environmental factors might be responsible for manifestation of disease (Concannon et al., 2009; Van Belle et al., 2011). MAP has also been indicated as an environmental trigger that might contribute to Type-1 Diabetes pathogenesis (Sechi et al., 2008; Naser et al., 2013). Singh et al. (2014) reported high burden of MAP in different kinds of infectious and non-infectious disease conditions e.g., diabetes, liver disorders, anemia, thyroid disorder, tuberculosis, typhoid, abdominal disorders, inflammatory illness and ion imbalance in human population from North India. The study aimed to screen human population for Type 1 Diabetes and Mycobacterium avium subspecies paratuberculosis in Chatarpur district of Madhya Pradesh state in India.
Materials and Methods
Collection of Samples
Informed consent from patients as well as other necessary clearances were procured before blood samples were drawn. A total of 111 human samples (88 serum and 71 blood) were collected from a ‘health camp’ organized by Gwalior Medical College at Chattarpur district of Madhya Pradesh to screen human population for diabetes and MAP infection. Of the 111 suspected human beings screened for diabetes, only 20 (18.0%) were positive using commercial kits (ACCU-CHEK blood glucose monitoring system, Roche).
Indigenous ELISA Kit
Human serum samples were screened by ‘Indigenous ELISA kit’ (Singh et al., 2007) for presence of MAP infection. Absorbance was read at 450 nm in iMark ELISA reader (BioRad). Defined positive and negative were simultaneously run. Optical density (OD) values were converted to sample to positive (S/P) ratios as per Collins (2002) to determine the status of MAP infection. Serum samples in positive and strong positive categories were considered positive for MAP infection. Ratio between mean OD of positive and negative control (≥ 4 times) was critical for calculating S/P ratio and categories were (0.00-0.09, negative; 0.10-0.24, suspected; 0.25-0.39, low positive; 0.40-0.99, positive; and 0.61-0.80, strong positive) as per Collins (2002).
S/P ratio=(OD 450nm of the sample - OD 450nm of the negative control) / (OD 450nm of the positive control- OD 450nm of the negative control)
IS900 PCR
MAP DNA was extracted from blood samples (n=14) and was subjected to screening by specific IS900 PCR following the protocol of Singh et al. (2010b). Briefly, in a volume of 12.5μl of 2X red dye PCR master mix (Genei), 1μl each of forward (10 pmol/μl) and reverse primer (10 pmol/μl), 7.5μl of nuclease free water and 3μl template DNA were added (25μl total volume). Thermal cycling conditions were: initial denaturation (94°C for 5 min), followed by 35 cycles of denaturation (94°C for 30 sec), annealing (64°C for 30 sec), extension (72°C for 30 sec), and a final extension (72°C for 10 min). Product size of 413 bp was considered positive after separation on 1.5% agarose gel electrophoresis. MAP IS900 primer sequences used as per Millar et al. (1996) were: P90 5’- GAAGGGTGTTCGGGGCCGTCGCTTAGG -3’ (Forward primer) and P91 5’- GGCGTTGAGGTCGATCGCCCACGTGAC -3’ (Reverse primer).
IS1311 PCR
IS900 PCR positive DNA were subjected to IS1311 PCR using forward (M56) and reverse (M119) primers as per Sevilla et al. (2005). Briefly, in a volume of 15μl of 2X red dye PCR master mix (Genei), 1μl each of forward (10 pmol/μl) and reverse primer (10 pmol/μl), 10μl of nuclease free water and 3μl of template DNA were added (total volume 30μl). Thermal cycling conditions were: initial denaturation (94°C for 5 min), followed by 35 cycles of denaturation (94°C for 30 sec), annealing (62°C for 30 sec), extension (72°C for 1 min), and a final extension (72°C for 10 min). Amplicon size of 608 bp on 1.5% agarose gel with ethidium bromide further confirmed presence of MAP infection.
IS1311 PCR-Restriction endonuclease analysis (REA)
IS1311 PCR-Restriction endonuclease analysis (PCR-REA) was carried out employing endonuclease enzymes (HinfI and MseI) as per Sevilla et al. (2005). Briefly, REA reaction was performed in a 30μl volume containing 20μl positive IS1311 PCR product, 5μl reaction 10X buffer and 1μl (5U) each of HinfI and MseI endonucleases (Fermentas), and reaction mixture incubated at 37°C for 2hrs. Digested products were visualized on 2% agarose gel electrophoresis and biotypes (band patterns) were interpreted as described by Whittington et al. (2001).
Statistical analysis
Proportional agreement (Kappa coefficient) between the tests was compared as per Arizmendi and Grimes (1995).
Results
Indigenous ELISA Kit
Of 88 serum samples screened, 34 (38.6%) were positive for the presence of MAP infection. Of the 20 confirmed ‘Type 1 Diabetes’ patients, 19 serum were collected and screened and of which 6 (31.5%) were positive for MAP infection by ‘Indigenous ELISA’ (Table 1 and 2). Comparative evaluation of ‘Indigenous ELISA’ with IS900 PCR showed that 14.7% human subjects were positive by ‘Indigenous ELISA kit’ as compared to IS900 PCR (20.5%). However, cumulatively, 16 (23.5%) human subjects were positive by the two tests. Proportional agreement between ‘Indigenous ELISA’ and IS900 PCR was substantial (64.7%) (Table 3 and 4).
IS900 PCR and Bio-typing
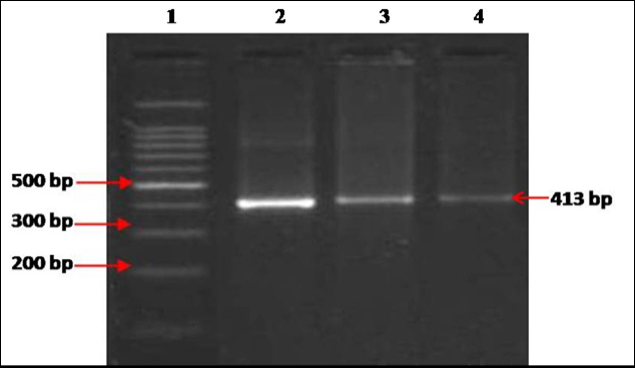
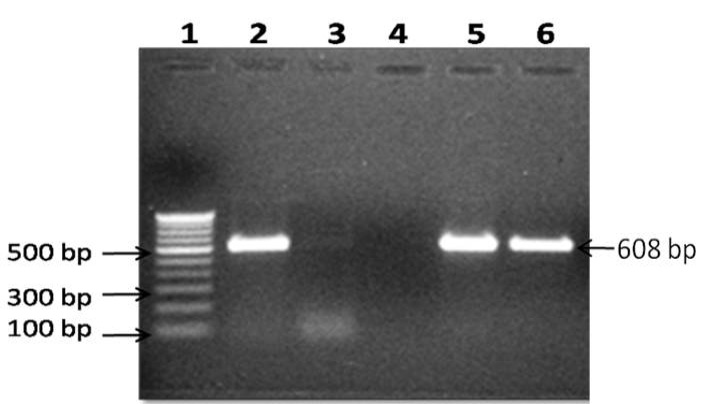
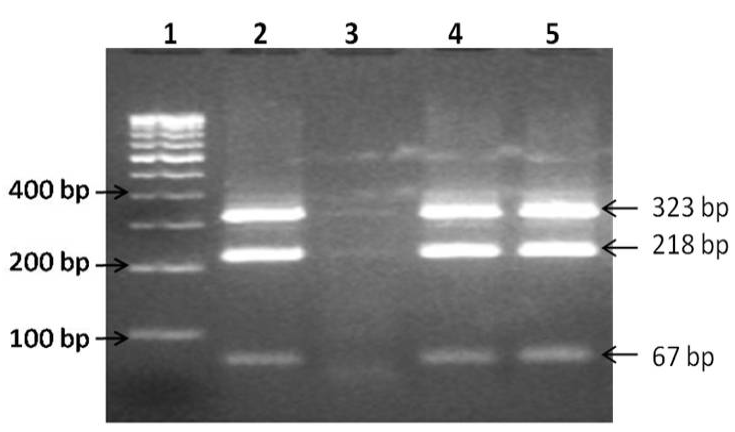
Of the 71 blood samples screened, 28 (39.4%) were positive in IS900 blood PCR (Figure 1). Of the 16 blood samples collected from confirmed type 1 diabetes patients, 7 (43.7%) were positive by IS900 PCR. Of 28 IS900 PCR positive samples, eight were subjected to IS1311 PCR and were positive (Figure 2). Bio-typing of 8 positive samples by IS1311 PCR-REA showed presence of highly pathogenic ‘Indian Bison type’ biotype (Figure 3).
Table 1: Comparative evaluation of S/P ratios of the results of Indigenous ELISA
|
S/P ratio |
Disease status |
Indigenous ELISA (n) |
|
0.00 - 0.09 |
Negative |
24 (27.2) |
|
0.10 - 0.24 |
Suspect |
16 (18.1) |
|
0.25 - 0.39 |
Low positive |
14 (15.9) |
|
0.40 - 0.99 |
Positive |
31 (35.2) |
|
1.0 - 10.0 |
Strong positive |
03 (3.4) |
|
Total human subjects |
88 |
*Positive and strong positive in S/P ratio taken as positive (38.6%); Figures in parentheses are percentage.
Lane 1: 100bp ladder, lane 2: Positive control (MAP DNA), lane 3-4: DNA samples
Lane 1: 100 bp DNA ladder, Lane 2: Positive control (MAP DNA), Lane 4: Negative control (miliQ water) and Lane 5-6: DNA samples positive in IS900 PCR
Lane 1: 100bp DNA ladder, lane 2: Positive control (MAP Indian Bison type DNA), lane 3: Negative control (miliQ water), lane 4-5: Digested DNA sample.
Table 2: Screening of type 1 diabetes and healthy suspects for MAP infection by Indigenous ELISA kit and IS900 PCR
|
Tests |
Healthy human suspects* |
Type 1 diabetes** |
||
|
Indigenous ELISA |
IS900 PCR |
Indigenous ELISA |
IS900 PCR |
|
|
Human beings (n) |
88 |
71 |
19 |
16 |
|
Positive n (%) |
34 (38.6) |
28 (39.4) |
6 (31.5) |
7 (43.7) |
Figures in parentheses are percentage;* Total -; ** Total diabetes patients - 20
Table 3: Comparative evaluation of Indigenous ELISA kit and IS900 PCR for the presence of MAP infection
|
Tests |
Combinations |
|||
|
Indigenous ELISA kit |
+ |
- |
- |
+ |
|
IS900 PCR |
+ |
- |
+ |
- |
|
Total (68) |
16 (23.5) |
28 (41.1) |
14 (20.5) |
10 (14.7) |
*Figures in parentheses are percentage; Agreement between ‘Indigenous ELISA kit’ and IS900 PCR – 64.7%
Table 4: Comparative evaluation of Indigenous ELISA kit and IS900 PCR for the presence of MAP infection in confirmed type 1 diabetes humans
|
Tests |
Combinations |
|||
|
Indigenous ELISA kit |
+ |
- |
- |
+ |
|
IS900 PCR |
+ |
- |
+ |
- |
|
Total (15) |
1 (6.6) |
4 (26.6) |
7 (46.6) |
3 (20.0) |
*Figures in parentheses are percentage; Agreement between ‘Indigenous ELISA kit’ and IS900 PCR – 50.0%
Discussion
MAP is the etiological agent of paratuberculosis, a chronic granulomatosis enteritis in domestic and wild ruminants including primates and human beings (Chiodini et al., 1984; Singh et al., 2010a, 2011, 2014). Recent Indian studies report sharp increase in bioload of MAP in domestic livestock species (Singh et al., 2013; Singh et al., 2014). A sub-clinically infected animal excrete huge quantities of bacilli in feces, milk, semen etc., and MAP is known to survive pasteurization temperature (Grant et al., 2001; Shankar et al., 2010). Therefore, high prevalence of MAP has been reported in raw milk, pasteurized milk and milk products (Ice cream, cheese, etc) in many countries including India and is a serious public health concern worldwide (Grant et al., 2001; Ikonomopoulos et al., 2005; Slana et al., 2008; Shankar et al., 2010). Singh et al. (2012) reported contamination of natural resources (soil and water) with MAP in North India. It is therefore important to restrict contamination of environment and natural resources by animal and human excreta laden with MAP in order to reduce transmission to animal and human population. To reduce contamination of human population with MAP, it is essential to reduce infection of MAP in animals, especially domestic livestock. Association between MAP and chronic inflammatory bowel disease (Crohn disease) has been reported and found to persist in cell wall-deficient form, escaping clearance by the host immune system (Sechi et al., 2008). Other reports suggested association of MAP with Type I diabetes (autoimmune disease) in patients from Sardinia (Italy), which is a MAP endemic and genetically isolated region (Rani et al., 2010; Cossu et al., 2011; Cossu et al., 2013, Bitti et al., 2012, Masala et al., 2014). Similarly country (India) possesses largest population of domestic livestock, which is endemic and has seen high surge in prevalence of MAP in animals and cases of Type 1 Diabetes in human beings. As per International Diabetes Federation about the number of diabetes patients may shoot up to 87 million in 2030 from current 50 million (Unwin et al., 2009). Rani et al. (2010) reported the role of gut microbiota, environmental micro-organisms and dietary factors as trigger the development of diabetes in genetically susceptible individuals. Present report tries to find a link between the two.
Present study report 31.5% prevalence of MAP in 20 confirmed type 1 diabetes patients using ‘indigenous ELISA’. Singh et al. (2008) reported high sero-positivity in CD patients (100.0%), animal attendants (75.0%) and apparently normal human beings (38.0%) in North India. Other workers also reported presence of MAP in human population particularly in patients of CD and diabetes (Greenstein et al., 2003; Bitti et al., 2012). Singh et al. (2014), after screening of 23,196 sera samples, by indigenous ELISA kits, reported over all 34.0% sero-prevalence of MAP in human beings suspected for different kinds of ailments. However, after screening of 9816 patients suspected for diabetes, 28.3% were positive. Detection of MAP specific proteins (MAP3733c and MAP3738c) establishes association of MAP with Type 1 diabetes (Cossu et al., 2013). However, Rani et al. (2014) contrary to the above findings, reported no difference in serum immune-reactivity to MAP cell lysate or MAP3738c protein between diabetic cases and healthy control. A finding which is hard to prove in view of high population densities of animals and human beings in India and high to very high prevalence of MAP in native livestock population, otherwise call for sero-reactivity in apparently normal human beings. Since large number of reports in India exist, wherein MAP has been recovered from these apparently normal or healthy persons, therefore doubt, whether MAP can cause any real health problem in human beings leave alone IBD or CD. Contrarily Singh et al. (2014), after mass screening of human serum samples, exhibited sero-prevalence of MAP in number of suspected infectious and non-infectious conditions in human beings.
In the present study, 39.4% blood samples were positive for MAP bacterimia using IS900 blood PCR. Of the 16 blood samples collected from confirmed Type 1 Diabetes patients, 43.7% were positive by IS900 PCR. Our study correlates with the finding of some authors that have claimed the association of MAP infection with autoimmune disorder viz. Type 1 Diabetes and thyroid disorder etc. Recent advancements in MAP research indicated presence and role of MAP in patients with diseases such as Type-1 Diabetes (Sechi et al., 2008), autoimmune thyroiditis (D’Amore et al., 2010), multiple sclerosis (Cossu et al., 2013), autism (Dow, 2011), sarcoidosis (EL-Zaatari et al., 1996) and autoimmune arthritis (Moudgil et al., 1997). Singh et al. (2014) screened 3093 blood samples of human beings suspected for different kinds of ailments for presence of MAP by IS900 PCR, 8.4% were positive for MAP infection. However, after screening of 451 blood samples of suspected diabetes patients, 4.8% were positive. Contrary to these findings Rani et al. (2014), though reported amplification of 16S rDNA in 66.0% healthy, 76.0% Type 1 Diabetes patients and 86.0% in type 2 diabetes patients, but none of these samples was positive in IS900 PCR. Similarly, Sasikala et al., 2009, reported MAP specific sequences in the biopsies of Crohn’s disease patients from Hyderabad.
Of 28 IS900 PCR positive DNA samples, eight were subjected to IS1311 PCR_REA and all had ‘Indian Bison type’ restriction pattern, which was major biotype of MAP infecting domestic and wild ruminants and also human population in North India (Singh et al., 2008; 2010a). Comparative evaluation of ‘Indigenous ELISA kit’ with IS900 PCR showed that 14.7% human subjects were positive by ‘Indigenous ELISA’ as compared to 20.5% by IS900 PCR. Cumulatively, 16 (23.5%) samples were positive by two tests indicating definite infection of the diabetes patients. Proportional agreement between ‘Indigenous ELISA kit’ and IS900 PCR was substantial (64.7%) (Table 3 and 4). Moreover, presence of same genotype (Indian Bison Type), in domestic livestock, wild ruminants, other animals, primates, milk and milk products and environment samples proves sharing of genotype and interspecies transmission. Rani et al. (2014), justified non-reporting of MAP in limited human sera samples due to fact that association of mycobacterial trigger with diabetes could be a population specific phenomenon, highly dependent on genetic repertoire and the environment of susceptible population. Singh et al. (2014) also reported difference in sero-prevalence MAP in human population between two adjacent districts; Agra (14.2%) and Mathura (35.4%), which may be due to chance, since population in two regions is very similar except Agra is growing metropolitan city and Mathura is still rural type. Contention of Rani et al. (2014) that high genetic susceptibility with respect to presence of SLC11A1 gene in type 1 diabetes affected population of Sardinia (Pugazhendhi et al., 2008), which also has high rate of MAP infection in their livestock (Rani et al., 2010) may not be true in India since large variability exists both in Indian population with respect to animals (species and breeds) and human beings.
Economic significance of MAP in dairy enterprise in general and domestic livestock in particular, combined with contamination of milk and milk products (Public Health significance), risk of human infection and increasing role of MAP in the development of Crohn’s disease, type 1 diabetes and other incurable diseases in human beings highlight the need to minimize chances of entry of bug in to food chain. Present findings are first report from India supporting the hypothesis of probable association of MAP and type 1 diabetes suggesting that there is sufficient ground to warrant focus on MAP and its possible involvement in triggering Type 1 Diabetes.
Acknowledgement
Authors are thankful to Indian Council of Agricultural Research (ICAR), New Delhi for providing financial assistance (Outreach project on zoonotic diseases) and Director, Central Institute for Research on Goats (CIRG), Makhdoom for providing laboratory facilities.
Conflict of Interest
No conflict of interest to declare.
References