Advances in Animal and Veterinary Sciences
Research Article
Efficacy and Safety of Natural Essential Oils Mixture on Tick Infestation in Dogs
Aziza M. Amer1*, Mohamed. M. Amer2
1Professor of Pharmacology, Faculty of Veterinary Medicine, Cairo, University, Egypt; 2Professor of Poultry Diseases, Faculty of Veterinary Medicine, Cairo, University, Egypt.
Abstract | In this study essential oils mixture (Lacecca®) was used for treatment (control) and prevention of ticks infestation in dogs (0.25 ml/kg b.wt) orally followed by water for 3 successive days followed by 4 weeks observation period. In infested oral treated dogs, ticks and skin affections (irritation and inflammation) were decreased and disappeared gradually by treatment. Alive tick number was decreased to 75% at 6 hours from the 1st dose to reach 99.42 % at 6 hours from the 3rd dose and 100% starting from 12 hours from the 3rd dose up to 28 days. Complete disappearance of ticks from tested dogs bodies at 7, 14 and 21 days after treatment. Oral dosing of essential oils mixture (Lacecca®) resulted in protection (100%) of dogs against ticks infestation, complete safety and no adverse effect on control and preventive group. Ticks infestation showed adverse effects on hematological values, decrease in Hb% (12.40±0.55), PCV % (37.13±1.47), TEC 106/ml 5.29±0.03 and increase in total leucocytic count (TLC 103/ml) 22.64±2.88 in infested dogs than in normal dogs. Infested treated group showed decrease in glucose (77.60±7.64mg/dl) and albumin (2.20±0.01g/l). Increase of total protein (7.79±0.03g/l), ALT (64.10±2.23 IU/l), AST (73.20±5.13IU/l) and ALP (140.10±2.07 IU/l00) levels than in normal dogs. Treatment with essential oils mixture improved health condition of tick infested dogs where all blood values and biochemical parameters returned to normal values. Preventive treatment with Lacecca® showed no bad changes in hematological values and biochemical parameters and it is safe in healthy and preventive group. The obtained results proved that essential oils mixture is safe, has no adverse effects and has high efficacy in both control (treatment) and prevention of tick infestation in dogs. Therefore, this study pointed out that essential oils mixture is a very promising safe and acaricidal agent for control and prevention of tick infestation in dogs.
Keywords | Essential oils, Ticks, Efficacy, Safety, Acaricid, Control, Prevention, Dogs
Received | December 31, 2019; Accepted | March 24, 2020; Published | March 20, 2020
*Correspondence | Aziza M. Amer, Professor of Pharmacology, Faculty of Veterinary Medicine, Cairo, University, Egypt; Email: aziza.mahrous@gmail.com
Citation | Amer AM, Amer MM (2020). Efficacy and safety of natural essential oils mixture on tick infestation in dogs. Adv. Anim. Vet. Sci. 8(4): 398-407.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.398.407
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Amer and Amer. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Ticks are the most common external parasites of dogs (Chomel, 2011; Dantas-Torres et al., 2012). Rhipicephalus sanguineus (R. sanguineus) also known as brown dog tick, kennel tick, or pantropical dog tick, is a worldwide species of tick and more commonly in warmer climates. Ticks transmit pathogenic agents including Babesia canis vogeli, Ehrlichia canis, Anaplasma platys and Hepatozoon canis to dogs and their owners (Beugnet and Marié, 2009; Chomel, 2011; Dantas-Torres and Otranto, 2016). Ixodes ricinus (I ricinus) is the main vector of Lyme borreliosis and tick-borne encephalitis (TBE) in human also its bite can cause meat allergy (Apostolovic et al., 2016; Hofhuis et al., 2016; Jaenson et al., 2012). I. ricinus has a wide range of time activity during the year (Beugnet and Chalvet-Monfray, 2013; Dantas-Torres and Otranto, 2013; Estrada-Peña et al., 2013).
Ticks infestation mostly associated with physiological changes can be diagnosed by hematobiochemical alterations (Sakina et al., 2012) and high risk of transmission of tick-borne pathogens (Halos et al., 2012; Fourie, 2015). These hematobiochemical parameters assist in diagnosing of infestation severity and helps in follow up of treatment of infested dogs.
In Veterinary practicing the synthetic neurotoxic insecticides (e.g. organochlorines, organophosphates and pyrethroids) are used as ectoparasiticides, but the development of insecticide resistance in addition to their adverse effect on human health (Kolaczinski and Curtis, 2004) and the environment (Ramwell et al., 2009). The restrictions on using of synthetic insecticides attract the researcher to develop botanical alternatives for ectoparasite management. Essential oils are approximately 20–80 different plant metabolites contain more than two or three major terpene or terpenoid components, which constitute up to 30% of the oil (Bakklai et al., 2008). In the last decade there has been an extensive research on the repellent and acaricidal effects of many essential oils against ticks. The majority of these researches have focused on species of Rhipicephalus and Ixodes (both: Ixodida: Ixodidae) ticks, largely in vitro. These repellent and acaricidal efficacy are often attributed to the oil’s major component(s); however, there is also evidence that oil components may work in synergy (Yang et al., 2003). Data on the effects of essential oils as tick treatments or repellents in vivo are still limited (Ellse et al., 2013; Ellse and Wall, 2014).
The mode of action of many essential oils or their components still under investigation, but there are many evidences of a toxic effect on the insect nervous system; terpinen-4-ol, a monoterpenoid in tea tree oil, inhibits arthropod acetylcholinesterase (Mills et al., 2004; Lopez and Pascual-Villalobos, 2010). While, the hydrophobic nature of the oils can cause mechanical effects on the parasite by disrupting the cuticular waxes and blocking the spiracles, leading to death by water stress (Burgess, 2009) or suffocation. Essential oil have antihistamine effect (Koh et al., 2002) and reduces the inflammation by acting as an anti-inflammatory action by increasing interleukin-10 production (Andrade et al., 2014; Aazza et al., 2014; Ali et al., 2015). Anti-inflammatory, wound healing and acceleration of healing processes of essential oils were due to presence of sesquiterpenes, saponins, and considerable levels of polyphenolic compounds (Duarte et al., 2009; Cascaes et al., 2015).
Essential oils are generally safe and have been approved as food additives and fall in the category of generally recognized as safe by the US FDA (Bilsland and Strong, 1990). Studies proved safety of these oils if used in aromatherapy (Oyedeji et al., 2009; Ali et al., 2015). Garlic oil is non-toxic to mammals (Mossa et al., 2018), has high acaricidal activity against eriophyid mites (Mossa et al., 2018) and arthropod pest (Dabrowski and Seredynska, 2007; Attia et al., 2011).
This study was carried out to assess the treatment and preventive efficacy of natural oils mixture; Garlic oil, Rapseed oil and Roship oil (Lacecca®) as well as, evaluation of it is safety by assay of hematology and biochemical parameters in healthy dogs and dogs suffering from tick infestation after recommended doses administration.
MATERIALS and METHODS
Animals
Forty (40) healthy mixed breed dogs, weighing 17 to 25 kg, and more than 6 months age of a breeds characterized by a fur of moderate hair length, offering chance for ticks to penetrate through the hair and being retained on the animals. Dogs were not exposed to ectoparasiticides for at least 3 months before start of the study. Physical and clinical examination was done of each dog to ensure that they are healthy before treatment.
Dogs housed in cages indoor animal house, at temperature (20-25 °C ± 4 °C) and photoperiod of 12 hs light: 12 hs darkness. Animals kept individually in cages 2.0X3.0 meters. Water was available ad libitum and an adequate amount of a commercial dog food towards their maintenance was provided daily. The study protocol was reviewed and approved by the institutional animal Care and Use committee, Faculty of Veterinary Medicine, Cairo University (vetCU06202019045).
Natural essential oils mixture (Lacecca®)
A 100% natural essential oils mixture (Lacecca®) for pets composed of (garlic oil 2.5%, Allicin 0.05%, rapeseed oil 8%). Obtained from Envisal Europe BV in Rotterdam. Lacecca® was applied as 0.25 ml/1kg b.wt via oral spray followed by water for 3 successive days/ month according to instructions.
Ticks collection
The major families of tick: hard ticks or Ixodes spp. (R. sanguineus) were collected from previously infested dogs. Ticks were identified in the laboratory using the taxonomic keys (Hoogstraal, 1956; Walker et al., 2003).
Animal grouping and treatment
Dogs divided into 4 groups (10 animals each). Animals in group 1 were kept as non-infested non-treated control dogs. Dogs of group 2 were kept as infested- untreated control dogs. Group 3 was infested and treated orally with essential oils mixture 0.25 ml/kg b.wt for 3 successive days/ month. Group 4 was pretreated with essential oils mixture (0.25 ml/kg b.wt) for 3 successive days as preventive before tick infestation. Animals in each group were weighed before and after treatment. Following essential oils mixture administration, the animals were offered food and closely observed for adverse reactions and clinically according to Food and Drug Administration (FAD) diagnosis.
Tick infestation and counts
Tick infestations were conducted on pentobarbital sedated dogs before exposure and 4 hours post exposure. Each dog was infested with 40 females and 10 males unfed adult ticks directly applied to their fur of the back, lateral side, and head (0 time). The tick numbers in treated and control group were counted at 6, 12 and 24 hours after 1st dose as well as at 3,7 14 28 days from start of treatment. Dog body parts (neck and head areas, inside legs, outside of legs and feet, tail and anal areas, outside and inside of ears) were examined and ticks were removed using forceps. The collected ticks were classified as dead or alive, attached or unattached and counted to assess the possible acricidal effect (Marchiondo et al., 2013).
The percentage efficacy was calculated as follows:
Efficacy (%) against ticks = 100 × (Mc –Mt) /Mc
Where;
Mc= Arithmetic mean number of live ticks (free or attached) on dogs in the negative control group (group 1) at a specific time point; Mt= Arithmetic mean number of live ticks (free or attached) on dogs in the natural essential oil’s mixture (oral sprayed) groups at a specific time point.
Clinical assessment
Dog observation was carried out according to the published description of the regional distribution and lesion appearance of FAD (Scott et al., 2001). The severity of the signs of FAD was graded on the following scale: very mild, mild, moderate, severe, very severe any non-FAD related skin disease was considered in resolution or interpretation of the signs of FAD (Fisara et al., 2015).
Assessment of essential oils mixture safety in treatment and prevention of tick infestation in dogs
Two blood samples were individually collected from dogs from radial vein using 24 gauge needles after treatment for evaluation of hematological analysis as well as biochemical, liver and kidney function parameters.
- 1. Blood samples were collected in vacuum tube containing anticoagulant (EDTA). Hematological estimations of TEC, PCV, mean corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin concentration (MCHC), Platelet count (Thous, TLC, DLC. Staff, Segminted). Lymphocytes, Monocytes, Eosinophils, Basophils calculated by standard laboratory procedures (Jain, 1986).
- 2. Blood samples were collected in 5 ml sampling tube capacity with gel clot activator, allowed for clot and serum was separated by centrifugation at 1000 g for 5 minutes. Diagnostic kits provided by BioScope diagnostics were used for determination of Glucose, Urea, Creatinine, Uric acid, Cholesterol, Tri glycerides, Bilirubin, AST, AST, ALP, Total protein and Albumin.
Statistical analysis
The groups were compared at each time point by a one-way Analysis of Variance (ANOVA). The level of significance at 5%.
RESULTS
Clinical assessment
Non-treated non-infested dogs showed normal health and skin and no signs of affections. Infested non-treated dogs showed skin lesions caused by tick bites in form of skin irritation and inflammation (Figure 1a). Skin lesions in infested treated dogs were decreased and gradually disappeared following and after administration of essential oils mixture (Figure 1b). The pretreated group (4) ticks were not attached to dog skin without detectable lesion.
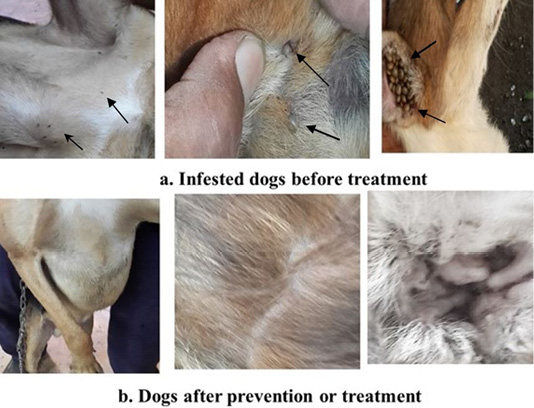
Figure 1: Showed tick infestation and skin affections in dogs. a: Before essential oils mixture treatment (Arows). b: After prevention or treatment.
All dogs skin lesions confined with active FAD, observed at 3,7,14 and 28 days (Table 4). All animals showed active FAD in day zero (100%). Treated group (3) showed decreasing of active bad lesion 40%, 10%, 0% and 0% at 3, 7, 14 and 28 days, respectively; while non-treated group (2) showed 100% active FAD at all intervals (Table 1). Animals of treated group showed inactive FAD 90%, 60%, 0% and 0% at 3, 7, 14 and 28 days, respectively; compared 0% in non-treated animals at all times.
Body weight
The recoded dogs body weights (Table 2) showed decrease in average body weight (ABW) of tick infested dogs at 28 days (1.93 kg/dog) as compared with increased ABW of treated dogs (2.15 kg/dog), while control negative group showed 1.15 kg/dog. Preventive treated group 4 showed
Table 1: Clinical assessment score of skin lesions of tick infested dogs treated with essential oils mixture.
| Group | Number of dogs (100% of total) | Day zero | 3 days | 7 days | 14 days | 28 days |
| 2 (Infested non treated) | Presence of active* FAD lesions | 10(100%) | ||||
| Presence of only inactive active * FAD lesions | 0(0%) | |||||
| 3 (Treated) | Presence of active* FAD lesions | 10 (100%) | 4 (40 %) | 1 (10%) | 0 (0%) | 0 (0%) |
| Presence of only inactive active * FAD lesions | 0 (0%) | 9 (90%) | 6 (60%) | 0 (0%) | 0 (0%) | |
Table 2: Difference in body weight/kg of dog groups before and 28 days after treatment.
| Gr No | Treatment |
Body weight/kg |
||
| Before treatment Mean ± SD | After treatment Mean ± SD | Difference | ||
| 1 | Control negative | 22.08 2.48 | 23.23 2.16 | 1.15 |
| 2 | Infested nontreated | 23.53 0.79 | 21.60 0.92 | - 1.93 |
| 3 | Infested treated | 21.60 0.92 | 23.75 0.81 | 2.15 |
| 4 | Preventive | 20.70 2.37 | 22.54 2.26 | 1.84 |
Table 3: Ticks numbers and efficacy % of essential oils mixture in R. sanguineus infested dogs after oral administration of 0.25 ml/kg b.wt followed by water for 3 successive days.
| Day after treatment |
Tick count at hours post dosing |
Mean of alive tick number |
Treatment efficacy % |
p-value |
|
| Group 2 (Infested nontreated) | Group 3 (Infested treated Treated) | ||||
|
1 (1st dose) |
+6 | 40 | 10 | 75 | <0.05 |
| +12 | 40.5 | 6 | 85.18 | <0.05 | |
|
2 (2nd. Dose) |
+6 | 35 | 2.5 | 92.85 | <0.05 |
| +12 | 34 | 1.5 | 95.58 | <0.05 | |
|
3 (3rd. dose) |
+6 | 35 | 0.2 | 99.42 | <0.05 |
| +12 | 34 | 0 | 100 | <0.05 | |
|
7 (from the 1st dose) |
+24 | 35 | 0 | 100 | <0.05 |
|
14(from the 1st dose) |
+24 | 38.5 | 0 | 100 | <0.05 |
|
21(from the 1st dose) |
+24 | 34.6 | 0 | 100 | <0.05 |
|
28 (from the 1st dose) |
+24 | 35.5 | 0.2 | 99.43 | <0.05 |
increase average body weight 1.84kg/dog than control negative (1.15 kg/dog). Generally general health condition, activity and body weight improved after essential oil mixture administration.
Table 2 difference in body weight/kg of dog groups before and 28 days after treatment.
Efficacy of essential oils mixture against tick infestation in dogs
The obtained results in Table 3, revealed gradual decrease in alive ticks number at 6 hours with rate of 75% from the 1st. dose to reach 99.42 % at 6 hours from the 3rd. dose and 100% starting from 12 hours from the 3rd. dose up to 28 days. Complete disappearance of ticks from the animal’s bodies at 7, 14 and 21 days after treatment. A complete preventive efficacy (100%) was obtained from using essential oils mixture against tick infestation in dogs.
Safety of essential oils mixture
Hematological values
The obtained results (Table 4) showed complete safety and no adverse effect of essential oils mixture on control and preventive group. Tick infestation in group 2 showed adverse effects on hematological values, decrease in Hb% (12.40±0.55), PCV % (37.13±1.47), TEC 106/ml 5.29±0.03 and increase in total leucocytic count (TLC 103/ml) 22.64±2.88 in infested dogs than in normal dogs 15.80±1.43, 47.06±0.77, 6.53±0.08 and 19.76±0.17, respectively. Treatment with essential oils mixture improved dog’s health condition and all blood parameters returned to normal values (Hb% (15.98±0.25), PCV% (47.93±0.01), TEC (106/ml) 6.58±0.08 and TLC (103/ml) (21.17±1.04). In preventive treatment group no changes in all hematological values was recorded (Table 4).
Table 4: Effect of essential oils mixture administration (0.25 ml/kg b. wt) for 3 successive days on Hematological values in healthy (prevention) and tick infested dogs (treatment) (n=10, Mean± SD).
| Parameter | Group (1) Control | Group (2) Tick infested | Group (3) Treated | Group (4) Prevention |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Hb (g/dl) | 15.80±1.43 | 12.40±0.55a | 15.98±0.25 a | 15.76±1.14 |
|
TEC 106/ml |
6.53±0.08 | 5.29±0.03a | 6.58±0.08 a | 6.36±0.79 |
| PCV (%) | 47.06±0.77 | 37.13±1.47a | 47.93±0.01 a | 47.09±0.50 |
| Mean Corpuscular Volume (MCV) fl | 72.50±4.61 | 70.40±5.61 | 73.40±1.48 | 74.80±5.51 |
| Mean Corpuscular Hemoglobin (MCH) pg | 25.50±0.01 | 23.70±1.79 | 25.60±1.08 | 27.20±1.06 |
| Mean Corpuscular Hemoglobin concentration (MCHC) % | 33.60±0.92 | 33.60±0.66 | 33.30±0.78 | 33.50±0.81 |
|
Platelet count (103/ml) |
165.40±6.88 | 244.70±12.32 a | 159.20±1.01 | 152.50±1.25 |
|
TLC (103/ml) |
19.76±0.17 | 22.64±2.88 a | 21.17±1.04 a | 20.26±1.06 |
| Neutrophils | 4.28±1.18 | 7.80±1.54 a | 6.95±0.15 | 8.45±0.01 |
| Lymphocytes | 87.00±1.23 | 65.00±1.20 | 83.20±0.14 | 81.20±1.50 |
| Monocytes | 4.00±0.07 | 3.12±0.03 | 4.30±0.18 | 4.00±0.06 |
| Eosinophils | 1.20±0.01 | 1.20±0.06 | 1.30±0.01 | 1.40±0.04 |
| Basophils | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
A, Significant at <0.05.
Table 5: Effect of essential oils mixture oral administration (0.25 ml/kg b. wt) for 3 successive days on dogs biochemical, liver and kidneys function parameters in healthy and tick infested, treatment and prevention. (Mean ± SD).
| Parameter | Control | Tick infested | Treatment | Prevention |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Glucose (mg/dl) | 99.10±7.92 | 77.60±7.64a | 97.40±2.84 a | 98.20±4.87 |
| Urea (mg/dl) | 28.10±9.09 | 29.60±2.87 | 30.50±7.26 | 28.10±7.30 |
| Creatinine (mg/dl) | 1.37±0.18 | 1.27±0.19 | 1.37±0.18 | 1.37±0.18 |
| U. acid (mg/dl) | 2.81±0.61 | 2.69±0.73 | 2.81±0.61 | 2.81±0.61 |
| Cholesterol (mmol/L) | 99.70±9.77 | 137.20±0.71 | 99.70±9.77 | 99.70±9.77 |
| Tri glycerides (mmol/L) | 63.50±13.48 | 72.30±9.76a | 63.50±13.48 a | 63.50±13.48 |
| Bilirubin 9mg/dl) | 0.34±0.03 | 0.33±0.03 | 0.34±0.03 | 0.34±0.03 |
| AST (IU/l) | 55.70±1.10 | 73.20±5.13a | 55.70±10.10 a | 55.70±10.10 |
| ALT (IU/l) | 53.30±9.11 | 64.10±2.23 a | 53.30±9.11 a | 53.60±11.23 |
| ALP (IU/l) | 79.40±1.45 | 140.10±2.07a | 77.90±1.81 a | 78.90 ±4.52 |
| Total protein (g/l) | 5.85±0.08 | 7.79±0.03 a | 5.79±0.63 a | 5.63±0.79 |
| Albumin (g/l) | 3.24±0.02 | 2.20±0.01a | 3.24±0.21 a | 3.31±0.21 |
A, Significant at P> 0.05.
Biochemical, liver and kidneys function parameters
The obtained results (Table 5) showed, in group 2 (infested treated) there was decrease in glucose (77.60±7.64mg/dl) and albumin (2.20±0.01g/l). Increase of total protein (7.79±0.03g/l), ALT (64.10±2.23 IU/l), AST (73.20±5.13IU/l) and ALP (140.10±2.07 IU/l00) levels than those of normal dogs, glucose (99.10±7.92mg/dl) and albumin (3.24±0.02g/l), total protein (5.85±0.08g/l) ALT (53.30±9.11 IU/l), AST (55.70±1.10 IU/l) and ALP (79.40±1.45 IU/l00), respectively. All biochemical parameters values in treated infested are returned to be close to control group dogs (glucose, 97.40±2.84 mg/dl) and albumin (3.24±0.21 g/l), total protein (5.79±0.63 g/l), ALT (53.30±9.11IU/l), AST (55.70±10.10 IU/l) and ALP (77.90±1.81 IU/l00). Parameters of preventive treatment group 4 showed no difference with those of control.
DISCUSSION
In the present study, the efficacy of essential oils mixture remained >92.85% against Ixodes Rhipicephalus at 12 hs count for a full month. This is the first time that oral use of essential oils mixture product for 3 days has been shown to provide such a rapid prophylactic and speed of kill against R. sanguineus effectiveness of acaricid at least 90% at the 6 h time point was calculated for a full month. The high efficiency of the studied essential oils mixture on ticks may due to acaricidal effect of used oils. These results supported by results Cetin et al. (2009, 2010). The acaricidal efficacy of essential oils against ticks was attributed to their volatile components (Tedonkeng et al., 2005; Cetin et al., 2009, 2010) and their synergistic effect (Lwande et al., 1999). Essential oils repellent effect may attributable to the presence of trace elements, nerolidol, where 0.001μL caused 98.3% repellency (Lwande et al., 1999). Previous researches recorded that essential oil of other plants repel ticks (Lwande et al., 1999; Jaenson et al., 2005; Tunón et al., 2006; Birkett et al., 2011).
The detected hemoglobin (Hb) value in tick infested groups ((12.40±0.55) was lower than in normal group (15.80±1.43), which is in agreement to the findings of Jani et al. (2004) who reported that canine cases of parasitic dermatitis had significantly lower Hb than normal dogs (Biswas and Roy, 2005). PCV was found significantly low as compared to healthy control group. The PCV values recorded in ticks infested (37.13±1.47) dogs was significantly low as compared to healthy dogs (47.06±0.77), these findings are in agreement with significant decrease in PCV level of Mange affected dogs with Sarcoptic scabie (Beigh et al., 2016).
The significantly decrease in RBC’s count at P<0.05 in infested dog group (70.40±5.61 per μl) as compared with healthy dogs group (72.50±4.61) is agree with report of Patel et al. (2005) and Nair and Nauriyal (2007). Leukocytosis in ectoparasite infestation may be explained as a part of inflammatory reaction directed towards ticks, fleas, lice and mites (Shah, 1994). Significant neutrophilia was found in ticks infested dogs 76.12±0.32 in comparison with healthy ones (71.12±0.51). Neutrophilia may be due to activation of defense mechanism of the body agonist the infection (Jain, 1986).
There was significant lymphopenia in tick infested dogs (65.00±1.20) as compared to healthy dogs (87.00±1.23). These results are in agreement with marked lymphopenia in skin affected infected dogs recorded by Nair and Nauriyal (2007). Dimri (1998) reported the infiltration of lymphocyte in the underlying malpighian and between the crust layer of skin leads to reduction in circulating lymphocytes. Monocytes and basophils do not show any significant difference as compared with healthy groups. Similar findings were reported by Sharma (2002). All the hematological values returned to the normal values after treatment with essential oils mixture indicating the protective and repairing effect of the studied essential oil mixture which supported by of garlic parts have antioxidant properties shown to be effective in reducing tributyltin (TBT) induced oxidative damage in vivo and in vitro (Liu and Xu, 2007) and had tissue protection against nicotine-induced oxidative damage (Augusti and Mathew, 1973; Fukao et al., 2004). Also, studies showed that Rapseed oil (canola oil) which is one of the studied oils mixture, contains different antioxidant substances (Vuorela et al., 2004; Loganes et al., 2019), vitamin E (Xu et al., 2011), with wide range variability in content and composition within rapeseed variees (Seker et al., 2008), carotenoids and phenolic compounds (Chen et al., 1996; Basta et al., 2011). Vitamin E as one of natural an-oxidant and radical scavenger is able to maintain the cellular integrity and promote the prevention and treatment of several inflammatory diseases (Combs and McClung, 1992; Morrissey et al., 1994). The most active component in the oil 92, 6-Dymethoxoy-4vinylphenol) acts as a potent oxidizing and nitrang agent (Wakamatsu et al., 2005). The Roship oil which in a member of used mixture is rich in phenolic compound, carotenoids, ascorbic acid, polyunsaturated fatty acids, linolenic acid, and phytosterols, mainly β-sitosterol which have antioxidant activity (İlyasoğlu and Arpa, 2017; Mármol et al., 2017).
Mean values of glucose of tick infested dogs (77.60±7.64) was significantly reduced as than healthy group (99.10±7.92). Similar results were previously reported in in parasitic infestation and skin infection in dogs (Jani et al., 2004; Sharma and Wadhwa, 2018; Chhabra et al., 2000).
The total protein values in tick infested dogs (7.79±0.03) were significantly higher than healthy dogs (5.85±0.08). This finding agrees with those recorded in skin affected dogs (Gupta and Prasad, 2001; Katariya et al., 2018). The increased total protein values was attributed to the increased immunoglobulins and circulatory immune responses (Aujla et al., 1999; Katariya et al., 2018). Albumin level was decreased in ticks infested nontreated dogs (2.20± 0.01) compared with level in non-infested (3.24±0.02) as reported by Patel et al. (2005). Reduction in albumin values may be due to increased protein catabolism from stress condition produced by various ectoparasites infestation as stated by Katariya et al. (2018).
The recorded increase in liver function enzymes values indicated some damage caused by the toxic byproducts of tissue breakdown. Activity of serum enzymes AST (73.20±5.130 and ALT (64.10 ±2.23) increased significantly in tick infested dogs than the healthy dogs AST (55.70±1.10) and ALT (53.30±9.11), respectively. Also, a significant increase in ALP (140.100±2.07) in infested dogs than (79.40±1.45) in healthy (Patel et al., 2005). However, no significant alteration was observed in Kidneys function parameters, urea, creatinine and uric acid these results indicated no detectable adverse effect of tick infestation. In the present study all the biochemical parameters returned close to the normal values and after treatment can be due to the antioxidant activities of essential oils components. All garlic components used in our oil mixture reported to be effective (Rabinkov et al., 1998; Miron et al., 2000; Fukao et al., 2004; Kuete, 2017; Satyal, 2017). Rapseed oil contains different anoxidant substances (Vuorela et al., 2004; Loganes et al., 2019), Vitamin E (Xu et al., 2011; Seker et al., 2008), carotenoids and phenolic compounds (Chen et al., 1996; Basta et al., 2011). The rosehip-seed oil has antioxidant activity (Grajzer et al., 2015; Ilyasoglu, 2017; Mármol et al., 2017; Da Silva et al., 2019), hepatoprotective, antioxidant and anti-inflammatory properties in kidneys disorders were described (Ashtiyani et al., 2013; Sadeghi et al., 2016; Zuk and Bonventre, 2016).
The obtained results showed gradual decrease of dog’s skin allergic reaction, inflammation and injuries caused by tick infestation and signs were completely relived after 7 days from treatment with essential oils mixture, these results can be attributed to the antihistaminic and anti-inflammatory and tissues repairing activities of the studied essential oils as a result of their antioxidant and anti-inflammatory activities. These results supported with the previous studies (Dirsch et al., 1998; Rabinkov et al., 1998; Miron et al., 2000).
No adverse effect of oral administration of the tested essential oils on dogs general health condition, hematological parameters as well as all biochemical parameters including liver and kidneys function parameters, these results proved safety of the essential oil mixture on dogs when used as treatment and prevention of ticks infestation. These results supported by the results obtained before. The essential oils are generally safe and approved as food additives by the US FDA (Bilsland and Strong, 1990) as well as aromatherapy (Oyedeji et al., 2009; Ali et al., 2015).
CONCLUSION
The obtained results indicated high efficacy of essential oils mixture in treatment and prevention of tick infestation in dogs, as well as they are safe and no any adverse effects were recorded, So, essential oils mixture is a very promising safe and acaricidal agent for tick infestation in dogs.
ACKNOWLEDGMENTS
Authors are greatly thankful to Envisal Europe BV in Rotterdam, G. Briesemann (Inventor), G. Blomsteel, (Assistant) and S. Giesemann (Assistant) for providing the tested materials and all scientific information.
AUTHOR CONTRIBUTION
AMA and MMA designed and planned this study, performed experimental work, samples collection and all laboratory tests. All authors shared, manuscript writing, drafted, revised the manuscript and approved the final manuscript.
Ethical approval
The research plan was approved from Cairo University institutional animal care and use committee (VET CU-IACUC) with approval number: VetCU06202019045.
Conflict of interest statement
The authors have no conflict of interests to declare regarding the publication of this paper. Also, the authors declare that the work was self-funded.
References






