Advances in Animal and Veterinary Sciences
Research Article
Effects of Spirulina platensis Algae Extract Early Feeding on Japanese Quail Embryos
Tareq Khalaf Hasan Aljumaily*, Ahmed Taies Taha
Natural Resources Research Center, University of Tikrit, Iraq.
Abstract | The blue-green algae (Spirulina platensis) is widely distributed worldwide. The nutritional value of Spirulina algae is well-documented. Spirulina has unique high protein content, about 50–70% by dry weight, and it is considered as a good source of essential amino acid. Additionally, Spirulina was recognised to have a wide range of essential nutrients, including essential fatty acids and polysaccharides, vitamins and minerals, and carotenoids.The aim of this study was to determine the injection effect of spirulina liquid extract on fertility and hatching traits, production performance, and some biochemical characteristics of quail eggs during the incubation period. Four hundred and fifty eggs were selected for hatching. The eggs were divided into three groups, and every group was treated differently. The results of the first treatment showed that there was a significant difference in the hatchability percentage of fertilized eggs. Furthermore, the percentage of failed eggs and weak chicks from the control treatment is almost significantly. The results of the second treatment showed that there was no effect on the average weight of the hatched chicks and feed conversion ratio. The third treatment showed a significant increase in the weight gain and feed consumed. It was noticed that the treatments did not have a notable effect on the relative weight of the liver, heart, and intestines. No significant differences were observed of physiological characteristics, as total protein concentration, enzymatic activity of GOT, GPT, and MDA level. The third treatment caused an increase in GSH glutathione level comparison with a control treatment. The injection of spirulina liquid extracts in late stages of incubation could improve the hatchability percentage chicks and their chances of survival. It can also strengthen the new hatch chicks immunity and antioxidants status.
Keywords | Spirulina platensis, Feed supplement, Growth, Quail production, Egg quality
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 23, 2018; Accepted | October 04, 2018; Published | November 06, 2019
*Correspondence | Tareq Khalaf Hasan Aljumaily, Natural Resources Research Center, University of Tikrit, Iraq; Email: [email protected]
Citation | Aljumaily TKH, Taha AT (2019).Effects of spirulina platensis algae extract early feeding on japanese quail embryos. Adv. Anim. Vet. Sci. 7(1): 30-37.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.1.30.37
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Aljumaily and Taha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The use of algae as a feed additive, not as a partial protein replacement, in laying hens is limited. Considering the cost-effectiveness and better egg production and quality, Spirulina could be used as a valuable natural feed additive. Therefore, the aims of the current study were to determine the effects of feeding different levels of supplemental algae (Spirulina platensis) on the performance, egg production, egg quality, blood profiles, and egg yolk cholesterol content of laying hens. (Selim et al., 2018).
The composition of the freshly laid egg is related to the maturity of the hatchling. Hatchling maturity differs considerably in different species (Nice, 1962), Hatchlings fall into four major categories based on criteria such as mobility, amount of down, and ability to feed themselves (Sotherland and Rahn, 1987), in Japanese quail the yolk makes up 50% by mass of the egg with 9.6 KJ/g of the freshly laid eggs (Pettit et al., 1984; Ramakrishna, 1999). The yolk supplied energy usage increases as the embryo growth and it is reflected in an increase in the oxygen consumption of the embryo. Most of the energy contained in the freshly laid egg is incorporated into the tissues of the hatchling. The embryo to form new tissues during growth and to meet the physiological demands for energy (maintenance) of the living embryo during incubation uses the remaining energy (Tareq, 2011; Park et al., 2015).
In the last three days of egg incubation. A decrease in the rate of fetal growth occurs related to the decrease rate of metabolic processes due to the decrease in the oxygen ratio and the depletion of growth sources in the egg. These difficult conditions accompanied by high temperatures require the introduction of certain nutrients, which will help the fetus to resist this tough environment. The first approach was the use of injecting eggs technique of hatching in ova injection, which has become widely used in hatcheries in many countries of the world (Anbarasan et al., 2011; Mariey et al., 2012; Zahroojian et al., 2013).
The growth of future chicks has improved without affecting the development of embryos during embryonic development or hatching (Ohta et al., 2001; Selim et al., 2018), and is one of the best ways to introduce nutrients to the fetus during incubation (Macalintal, 2012).
Feeding the embryo prior to hatching using nutrient injection technique is expected to increase the hatching rate and effectively assist the fetus’s fight for hatching by ensuring that the eggs emerge from the eggshell, develop the digestive system and increase body weight (Uni and Ferket, 2004). Bhanja and his colleagues (2007) emphasized that injecting of fertilized egg with nutrients (vitamins) is beneficial in improving body weight during the breeding period. There are many substances used in the feeding process to inject hatching eggs such as vitamins, carbohydrates,some mineral elements, and supplements. The utilization of natural food supplement produced from algae Esperolina, which is from green algae blue single cell Due to the presence of both chlorophyll (green) and phycocyanin (blue) pigments in its cells.
Spirulina (blue-green alga) is a high quality natural feed additive that can be used in animal and poultry nutrition. The blue-green algae (Spirulina platensis) used for hundreds of years as a food source for humans and animals due to the excellent nutritional value and high carotenoid content. Spirulina is relatively high in protein with values ranged between 55-65% and includes all of the essential amino acids (Bourges et al., 1971; Anusuya Devi et al., 1981; Jamil et al., 2015). Chickens receiving dietary Spirulina found healthier than their not supplemented counterparts (Venkataraman et al., 1994). This is due to increased functionality of macrophage and overall mononuclear phagocyte system indicative of enhanced disease resistance with increased dietary Spirulina levels in chickens (Qureshi et al. 1996; Al-Batshan et al., 2001). Qureshi et al. (1996) found improved chicken health with low dietary Spirulina levels of 10 g/kg in the ration, indicating greater production cost efficiency.
The available energy content of Spirulina was determined to be 2.50- 3.29 kcal/gram and its phosphorous availability is 41% (Yoshida and Hoshii, 1980; Blum et al., 1976). In addition, Spirulina algae are rich in thiamin, riboflavin, pyridoxine, vitamin B12, vitamin C and carotenoids and used throughout the world as a feed component in broiler and layer diets to enhance yolk color and flesh (Colas et al., 1979; Brune, 1982; Ross and Dominy, 1985, 1990). In addition, it is rich in nutrients such as vitamins, amino acids, gamma linoleic acid, phycocyanins, tocopherols, chlorophyll and β-carotenes (Abd El-Baky et al., 2003; Khan et al., 2005).
The goal of this study was to investigate the effects of injectionquail eggs with Spirulina water extract on fertility, hatching, productive characteristics and physiological performances of quailchick. The second section of this paper includes the necessary materials and methods. The results of quail hatching egg illustrated in section three. Finally the paper conclusions were appeared in the section four.
MATERIALS AND METHOD
In this study, 450 fertilizedeggs of quail were obtained from the animal farm/ College of Agriculture / University of Tikrit on 1/9/2016. The flock was nine weeks old and with sexing ratioof one male for every two females. The eggs were selected for hatching in terms of the average weight of the egg is 11-12 grams, and they were clean and free from defects in shape, calcification.
The injection done onday fourteen (which is the transition day from incubation to hatching).The injection was carried out in the air chamber of the egg after the puncture area was sterilized with ethyl alcohol at a concentration of 70%, and 1.00 mm diameter drill was used for piercing the eggshell. The solution injected using a medical syringe of 1.00 ml size (Gauge 26). When the injection completed, the hole sealed by paraffin wax.
Preparation of Spirulina Liquid Extract
Spirulina liquid extract was prepared in two concentrations: the first was by dissolving 1% concentration distilled water, and the second concentration by dissolving 2% concentration distilled water, (Spirulina tablets produced by DXN company in Malaysia). The eggs treated with three treatments. Each treatment contained 150 hatch eggs distributed on three replicates of 50 eggs each. The eggs were treated as follows:
First treatment (T1): Control treatment, includes eggs that injected with 50 microliters of sterilized saline (0.85%).
Second treatment (T2): Includes eggs that injected with 50 microliters per egg at 1% concentration.
Third treatment (T3): Includes eggs that injected with 50 microliters per egg at 2% concentration.
After the hatching stage completed, the hatching rate and the percentage of the hatched eggs to the fertilized eggs, the proportion of dead embryos and the proportion of poor chicks were calculated according to the method suggested by Pedroso et al. (2006).
Weight of Hatching Chicks
All the chicks were weighed in each replicate for each treatment to determine the weight of each chick. The resulting post hatch chicks from the first experiment were raised in ground cages made of metal wires with dimensions of 100 x 100 x 60 cm. The total number of cages for the entire experiment was 12 cages, as of three cages for each treatment. Each cage designed to hold 20 chicks within its boarder. The floor of the cage laid out with rice husks, and each cage is provided with a hanging cylindrical water container and longitudinal feeder, providing the hall with all basic requirements of lighting, ventilation, humidity according to the age of the chicks. There was a free access for the chicks to water and food, and the feeding was in two rations, as shown in Table 1. The rations calculated according to NRC (1994).
Table 1: Ingredient composition (g/kg) Of the basal diets (as fed basis)
| ingredient | 0 – 21 d (%) | 21 – 35 d (%) |
| Corn | 44.83 | 44.7 |
| Soybean meal | 37.52 | 28.1 |
| Wheat | 8.045 | 18.305 |
| Di calcium Phosphate | 1.1 | 1.02 |
| Soybean oil | 4.46 | 4.60 |
| Limestone | 1.0 | 0.31 |
| DL-Methionine Trace | 0.18 | 0.09 |
| mineral–vitamin premix1 | 0.365 | 0.375 |
| Premix | 2.50 | 2.50 |
| Energy | 3069.69 | 3168.21 |
| Protein | 22.74 |
19.39 |
1Supplied per kg of diet: vitamin A, 3,600,000 IU; vitamin B 12, 6 mg; vitamin B1, 720 mg; 2640 mg vitamin B2; 4000 mg nicotinic acid; 1200mg vitamin B6; 400 mg folic acid; vitamin D3 8000 IU; vitamin 7200 IU; 800 mg vitamin K3; biotin 40 mg; antioxidant 100,000 mg; choline chloride 50000 mg; Mn 4000 mg; Zinc 33880 mg; Iron 20,000 mg; Cu, 4000 mg; I, 400 mg; 80mg, Se; 2 SAFIZYME® GP800 enzyme (a multi-enzyme product) contents: 0.0 and 3g/kg. The added enzyme is a commercial product, Safizym GP 800 that has 3500 U ß –glucanase, 600 U xylanase and 10.2 U cellulose activity per g of product.
The treatments were as follows:
First treatment: includes chicks hatched from eggs, which were injected with a physiological solution.
Second treatment: includes chicks hatched from eggs, which were injected with 1% concentration of Spirulina water extract.
Third treatment: includes chicks hatched from eggs, which were injected with 2% concentration of Spirulina water extract.
Productivity Traits
Include the calculation of both body weight, weight gain, feed consumption, and food conversion ratio, calculated weekly and as indicated by (AL-Mashaikey and Taha, 2015).
After 35 days of rearing for the hatching chicks, six birds selected randomly and slaughtered from each treatment for the purpose of studying and calculating the relative weight of the heart, liver and intestines.
Physiological Traits
Three birds randomly selected from each treatment to studying the physiological blood traits. This was done by cutting the jugular vein of each of them and obtaining the blood, which was then preserved in tubes containing gelatin gel. Obtain a serum to study each of the following traits:
The concentration of the total protein was measured by a testing kit from Biolabo SA Co. and the instructions attached to the kit. Glutamate Oxaloacetate Transaminase (GOT) and Glutamate Pyruvate Transaminase (GPT) were also measured using a kit the Egyptian manufacturer Spectrum according to the manufacturer instructions (Haussmann et al., 2011).
The antioxidant state was estimated by measuring the level of Malondialdehyde (MDA) using the reaction of Thiobarbituric acid (TBA) which were modified by (Bellairs and Osmond, 2014) and the measuring the level of Glutathione (GSH) using the method of the detector Ellman that was modified by Zamili and others.
Statistical Analysis
Complete Random Design (CRD) and the General Linear Model method were used to analyze the results of the study and compare the means, which are embedded in the Statistical Analysis Software package (SAS, 2005) to assess the effect of the external factors. Duncan test (Duncan, 1955), performed to determine the significance of the differences between the means of the factors on the studied traits at the probability (p < 0.05).
RESULTS
Fertility and Hatching Traits
The results of Table 3 indicate that the injection of quail hatching eggs by different concentrations of spirulina liquid extract resulted in a significant increase in the hatch ability compared to the control treatment, and the use of
Table 2: Effect of injection of quail hatching eggs by different concentrations of spirulina liquid extract on Fertility and Hatching traits.
| Traits | Treatments | ||
| Control (T1) | AS1(T2) | AS2(T3) | |
|
Hatchability % |
0.56 ± 50.33 C |
58.32±0.41 A |
54.32±0.52 |
|
Hatching eggs from fertile eggs % |
77.34 ±0.19 B |
79.91± 2.1 A |
82.11±1.84 A |
|
Pipecking eggs % |
2.08 ± 1.28 |
2.03 ±1.27 |
2.11 ± 1.15 |
| Weak chicks |
1.280.18 ± |
1.27 ±0.11 |
1.15 ±0.22 |
Values in the same row with no common superscript differ significantly at ( P<0.05).
Control: The eggs that injected with 50 microliters of with sterilized saline (0.85%).
AS1: The eggs that injected with 50 microliters per egg of (dissolving 1g of spirulina tablets in 100 ml distilled water)
AS1: The eggs that injected with 50 microliters per egg of (dissolving 2g of spirulina tablets in 100 ml distilled water)
Table 3: Injection embryos birds quail at different levels of aqueous extract of the preparation spirulina in some productive traits effect (Values Are Averages ±Standard Error)
| Age | One day | First week | Second week | Third week | Fourth week | Fifth week |
| Transactions | Weekly live body weight (g) | |||||
| T1 | 7.16 ± 0.20 |
29.6 ± 0.88 |
59.4 ± 1.3 | 91.46 ± 1.3 | 124.7 ± 1.8 | 158.6 ± 1.1 |
| T2 | 7.2 ± 0.15 | 30.3 ± 0.80 | 59.0 ± 1.4 | 92.0 ± 1.0 | 125.5 ± 1.2 | 155.5 ± 2.6 |
| T3 | 7.33 ± 0.21 | 31.6 ± 0.33 | 59.9 ± 0.96 | 93.1 ± 1.0 |
126.5 ± 1.4 |
173.0 ±2.3 |
| Weekly weight increase ratio(g / bird) | ||||||
| Duration | First Week | Second Week | Third Week | Fourth Week | Fifth Week | Total Duration |
| T1 | 22.4± 0.6 | 29.8 ± 0.5 | 31.9 ± 0.5 | 33.3 ± 0.3 |
33.8 ± 0.7 b |
151.4 ± 0.9 b |
| T2 | 23.1 ± 0.7 | 28.6 ± 0.7 |
33.0 ± 0.7 |
33.5 ± 0.5 | 29.9 ± 2.1 b |
148.3 ± 2.6 b |
| T3 | 24.3 ± 0.2 | 28.2 ± 0.6 | 33.2 ± 0.1 |
33.4 ± 0.4 |
46.5 ± 2.8 a | 165.6 ± 2.4 a |
| Amount of feed consumed (g) | ||||||
| T1 |
51.3 ± 1.0 |
75.2 ± 0.9 a | 93.5 ± 1.7 | 113.7 ± 0.4 |
136.7 ± 1.5 b |
470.6 ± 0.7 b |
| T2 | 52.1 ± 2.2 |
72.7 ± 0.8 ab |
95.5 ± 2.0 | 11 5.0 ± 2.4 |
121.2 ± 9.1 b |
456.6 ± 7.1 b |
| T3 | 54.9 ± 0.5 | 70.5 ± 1.0 b | 94.9 ± 1.5 | 112.0 ± 2.7 | 185.2 ± 8.8 a |
517.6 ± 5.1 a |
| Food Conversion Efficiency | ||||||
| T1 | 2.29 ± 0.03 | 2.52 ± 0.03 | 2.92 ± 0.03 | 3.41 ± 0.02 | 4.04 ± 0.04 | 3.10 ± 0.02 |
| T2 |
2.25 ± 0.03 |
2.54 ± 0.04 | 2.89 ± 0.02 | 3.43 ± 0.03 |
4.04 ± 0.05 |
3.07 ± 0.02 |
| T3 | 2.26 ± 0.03 | 2.50 ± 0.03 |
2.85 ± 0.03 |
3.35 ± 0.05 | 3.99 ± 0.08 |
3.12 ± 0.03 |
Different English letters within the columns indicate significant differences at the level of the probability (p≤ 0.05)
T1 : Control treatment (chick ensure the result of eggs injected physiological saline)
T2 : Chick embryos resulting from eggs injected with a solution ensuring that
extract Alaspirolina concentration of 1 g / 100 ml of distilled water (AS1).
T3 : Chick embryos resulting from eggs injected with a solution ensuring that extract Alaspirolina concentration of 2g / 100 ml of distilled water (AS2).
higher concentration resulted in a significant increase (P≤0.05) in compare to the Lower concentration. In addition, the second and third treatments resulted in a significant increase in the percentage of hatching eggs from fertilized eggs when compared with the control treatment. In addition, no significant differences observed between the treatments effect on the percentage of the pipecking eggs and the weak chicks.
Productivity Traits
The injection of hatching eggs by different concentrations of spirulina liquid extract did not have any significant effect on the weight of the new hatching chicks at day one and during the five weeks of raising. As for the weekly weight gain, we noted that the birds produced from eggs that were injected with spirulina liquid extract in the third treatment show more superiority in comparison to the first and second treatment at the age of five weeks and during the total period of the experiment. Same results achieved regarding the feed consumption, where it is observed that feed consumption has decreased at the second week of study in the third treatment birds compared with the control treatment. However, it has been noticed that the treatments have not shown any significant effect on the efficiency of feed conversation during the entire study.
Internal Organs
The results of Table 4 indicate the effect of injection of the embryos in their later stages with different concentrations of spirulina liquid extract in the relative percentage and relative weight of some internal organs. There was no significant effect for these treatments in the relative percentage and relative weight of the liver, heart and intestines.
Table 4: Injection Embryos Birds Quail at Different Levels of Aqueous Extract of the Preparation spirulina in Some Internal Organs.
|
Qualities
Treatments |
Percentage of compliance | Relative weight of the liver | Relative weight of heart | Relative weight of intestine |
| T1 | 68.5 ± 1.0 | 2.71 ± 0.1 | 0.8 ± 0.08 | 6.3 ± 0.5 |
| T2 | 69.1 ± 0.7 | 2.8 ± 0.2 | 0.7 ± 0.05 | 5.2 ± 0.2 |
| T3 | 67.5 ± 1.1 | 2.8 ± 0.2 | 0.7 ± 0.02 | 6.0 ± 0. 2 |
| M. P | NS | NS | NS |
NS |
NS: no significant
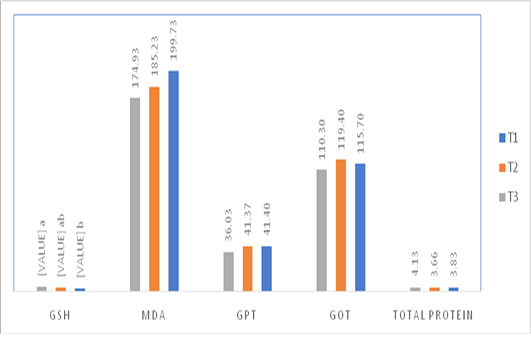
Figure 1: Effect of injection of quail hatching eggs of different concentrations of the spirulina liquid extract on some blood biochemical traits.
ABValues on the columns with no common superscript differ significantly at ( P<0.05).
Control: The eggs that injected with 50 microliters of with sterilized saline (0.85%).
AS1: The eggs that injected with 50 microliters per egg of (dissolving 1g of spirulina tablets in 100 ml distilled water)
AS1: The eggs that injected with 50 microliters per egg of (dissolving 2g of spirulina tablets in 100 ml distilled water)
Physiological Traits
The injection of quail fertile eggs different concentrations of spirulina liquid extract did not have any significant effect on some of the blood biochemical traits such as the total protein concentration, GOT, GPT, and MDA level. However, the injection with high concentration of spirulina liquid extract caused a significant increase in the level of GSH in compare with control treatment, as shown in Figure 1.
Discussion
The hatching rate is an important measure through which the amount of hatched chicks is expressed and depends on several factors, including males and females, where females produce eggs after fertilization with male sperm, as well as provide them with nutrients, and their needs to grow and develop (Selim et al., 2018). The hatching period (the last three days of incubation) is a critical period for the embryo life. The vital organs are developed in addition to the physiological and metabolic changes, including increasing the secretion of adrenal gland hormones to meet the needs of non-carbohydrates, primarily fats which represent the main source of energy during hatching and the following 72 hours (Ibrahim et al., 2015).This process increases the chance of embryos being exposed to oxidative stress caused by the hatching process and metabolic changes (Abdel-Daim et al., 2015).
The spirulina liquid extract can be used to inhibit peroxidation because of its content of nutrients, vitamins and antioxidants (Miranda et al., 1998). It was noted that the spirulina liquid extract has high content of vitamins dissolved in fat, such as vitamin A, E, and C. Vitamin A helps in recreate RNA, and DNA, in addition to his role in improving metabolic processes and cellular differentiation of fetus during embryonic development (Sporn et al., 1994). As for vitamin E, it works as an antioxidant to prevent damage of protein, which leads to better digestion and utilization of nutrients (Khaligh et al., 2017), and it also plays a role in maintenance and preservation of nitric oxide from the harmful effects of free radical, which helps stimulate cellular metabolism (Leornzoni and Ruiz-Feria, 2006). Nitric oxide also helps to increase muscle growth, regulate blood flow, and differentiate muscle cells (Stamler and Meissner, 2001).
The spirulina liquid extract contains vitamin D, which modifies the rate of cloning of target genes from the major genes, which are responsible for the biological response that regulates cell growth and reproduction (Adriana et al., 2005). Also, pointed out to one of the potential roles of vitamin 1.25 - (OH) 2D3 to support growth by stimulating the proliferation of cells and differentiation in the stages of growth and also works to increase the cell activity of the intestines mucosa as well as vitamin D3 is associated with vitamin receptors found in the DNA molecule, which simulate RNA and protein synthesis (Binderman and Weisman, 1983).
The absence of significant differences in the carcass weight and relative weights of some organs can be considered as an indication that the percentage used did not cause any damage to the embryos. Moreover, no significant differences were observed in physiological blood parameters, such as total protein and the enzymes of the amine group and the level of Glutathione. It was noticed that the injection treatments had a significant effect on decreasing the level of MDA in the serum (Anbarasan et al., 2011; Mariey et al., 2012; Zahroojian et al., 2013).
This is an important indication that the injection of hatching eggs with the extract of spirulina reduced oxidative stress during the hatching period. This means that the embryos, which obtained the spirulina liquid extract have improved its immunity against oxidative stresses and their damage, which allow the embryos to redirect the available energy towards the hatching process and maintenance of vital organs (Kotrbáček et al., 2013; Selim et al., 2018).
CONCLUSION
Injection of hatching eggs with the extract of spirulina reduced oxidative stress during the hatching period. This means that the embryos, which obtained the spirulina liquid extract have improved its immunity against oxidative stresses and their damage, which allow the embryos to redirect the available energy towards the hatching process and maintenance of vital organs.
ACKNOWLEDGEMENTS
All the authors of the manuscript thank and acknowledge their respective University and Natural Resources Research Center as well as Animal Production Department in Agricultural Colleges.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors contribution
Both authors contributed significantly to the paper. The principle/corresponding author Tareq KH. Aljumaily along with Ahmed T. Taha carried the experimental trial and compiled the results, Sample collection, and manuscript preparation. Both authors read and approved the final manuscript and wrote the paper.
REFERENCES






