Advances in Animal and Veterinary Sciences
Research Article
Detection of Toxoplasma gondii in Aborted Women and Meat of Slaughtered Sheep and Cattle in Sohag City, Upper Egypt
Nahed Mahmoud Abdel-Aziz1, Alshimaa A. Hassanien2*, Mohsen Ibrahim Arafa3
1Food Hygiene Department, Faculty of Veterinary Medicine, Sohag University, Egypt; 2Zoonoses Department, Faculty of Veterinary Medicine, Sohag University, Egypt; 3Parasitology Department, Animal Health Research Institute, Assiut Branch, Egypt.
Abstract | This work aimed to detect Toxoplasma gondii (T. gondii) and factors associated with infection in aborted women and meat of some slaughtered ruminants in Sohag city as a source of human infection. Ninety blood samples and 90 placenta tissue were collected from aborted women admitted to governmental hospitals in Sohag city. Animals’ samples included 96 meat samples (48 sheep and 48 cattle) slaughtered in Sohag city abattoir. ELISA and n PCR (Nested Polymerase Chain Reaction) were used to detect T. gondii infection in aborted women samples. Data was collected from aborted women through a standard form. While Latex agglutination test and microscopical examination were used in meat samples examination. Also, mice inoculation was used to evaluate the effect of meat freezing on the viability of T. gondii. Results indicated that 35.6% of aborted women serum represented positive results with ELISA and the majority possesses IgG (30%), 3.33% had IgM and 2.22% had both IgM and IgG. n PCR was used for detection of T. gondii in blood and placenta tissue of seropositve cases; T. gondii was detected in four and 25 of blood and placenta tissue of aborted women, respectively. Owing to factors associated with infection; aborted women from rural communities, those eating undercooked meat and who ignore periodical hand washing more susceptible to infection (P <0.01). 45.8% and 31.3% of sheep and cattle meat (mutton and beef) were positive for T. gondii, respectively. Viability of T. gondii was lost after meat freezing at – 20ºC for ten days. Strict implementation of supervision plan to eliminated infection in animals, food and human is important for preventing the risk of zoonotic transmission and human infection with T. gondii.
Keywords | T. gondii, Nested PCR, ELISA, Aborted women, Sohag city, Latex agglutination, Mutton, Beef
Received | March 22, 2020; Accepted | May 27, 2020; Published | June 02, 2020
*Correspondence | Alshimaa A. Hassanien, Zoonoses Department, Faculty of Veterinary Medicine, Sohag University, Egypt; Email: [email protected]
Citation | Abdel-Aziz NM, Hassanien AA, Arafa MI (2020). Detection of Toxoplasma gondii in aborted women and meat of slaughtered sheep and cattle in Sohag city, upper Egypt. Adv. Anim. Vet. Sci. 8(6): 680-686.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.6.680.686
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Abdel-Aziz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Toxoplasma gondii (T. gondii) is a worldwide protozoan parasite affects animals, birds and humans. Felids considered the main reservoir which shedding non sporulated oocyst in their feces, then sporulation will exist in the environment representing an important source of infection especially for grazing animals. In animals, sheep represent the highest infection rate with tissue cyst containing bradyzoites in muscles and brain (Tenter et al., 2000). Human get infected by ingestion of undercooked or raw meat containing viable tissue cyst, water and food contaminated with oocyst or through placenta (Mahmoud et al., 2012). Human infection with toxoplasmosis causing abortion in women, fetal abnormalities, stillbirth and may cause a severe complication in immunocompromised patients (EFSA, 2011). Therefore, using rapid and sensitive diagnostic methods of T. gondii in animals and human will stop disease complications and strict application of food safety strategies to prevent T. gondii transmission through food (Villena et al., 2012). Several diagnostic methods were used for T. gondii detection worldwide as histopathology, Polymerase chain reaction (PCR) and serelogical tests as ELISA, latex agglutination test (LAT), modified agglutination test (MAT), western blot (WB) and IHA (indirect hemagglutination test) (Dubey, 2009). Meat juice serology considered an excellent diagnostic method of T. gondii infection in different animal species at slaughter (Bacci et al., 2015). Using serological test beside nested PCR will be useful in detection of T.gondii (Fallahi et al., 2014). Reducing human infection from infected meat was done through tissue cyst destruction by irradiation, freezing or cooking (Kotula et al., 1991). Several studies worldwide proved that Toxoplasmosis infection in animals and human varies between different countries due to several factors as geographical distribution, socioeconomic status, environment and public health habits. So, increasing knowledge about infection risk factors possesses a major tool in effective control program and improving health of communities (Remington et al., 2001; Pappas et al., 2009). From several researches in Egypt, T. gondii considered a serious problem in both animals and humans as it was detected in several animal species, birds and food and causes a great health problem in humans especially pregnant women (Sukthana, 2006; Dubey, 2010). Therefore, our goal was to detect T. gondii incidence in meat of slaughtered sheep and cattle in Sohag city and in aborted women, also detect the effect of freezing on the viability of T. gondii in meat samples in addition to put our hands on the risk factors associated with aborted women infection.
MATERIALS AND METHODS
I-Human samples and data collection
90 blood samples and placental tissue were collected from aborted women admitted to governmental hospitals in Sohag city. Blood samples were divided into two portions (serum for antibodies detection using ELISA and whole blood for detection of T. gondii DNA using n PCR). While placenta tissue samples were collected in sterile cups and send to the laboratory for DNA extraction. Data were collected from aborted women after informed consent through a standard form including age, residence, trimester, abortion history, farm animal contact, cat contact, food habits (eating undercooked meat and eating raw vegetables) and personal hygiene habit (periodical hand washing).
Serological examination of aborted women samples
ELISA test was applied on women serum samples for detection of Toxoplasma IgM and IgG using test kits from Precheck Bio, USA (Saki et al., 2015).
DNA extraction and Nested PCR
DNA was extracted from blood and placenta tissue of aborted women which interpreted as positive by ELISA test following the manufacture protocol of DNA extraction kit of Qiagen, Germany. Nested PCR was done through two reactions; the first PCR product was amplified based on (Su and Dubey, 2009) using external primers; forward 5-GACGTCTGTGTCACGTAGAAAG-3 and reverse 5-CTGCAGACACAGTGCATCT GG ATT-3. The second reaction for PCR product 162 bp was amplified using internal primers; forward 5-AGGAGAGATATCAGGACTGTAC-3 and reverse 5- GCGTCGTCTCGTCTAGATCG-3. PCR reactions was done following Matin et al. (2017) with changes in cycling conditions as one cycle for five minutes at 95 ºC, then 30 cycles for one minute at 94 ºC, two minutes at 56 ºC and 72 ºC for five minutes using Bio-RAD, USA thermal cycler. The product was detected using agarose gel 2% stained with ethidium bromide and visualized by UV transilluminator from Biometra.
Statistical analysis
SPSS version 14 (SPSS, Inc., USA) was used to find the relation between T. gondii infection and aborted women characteristics.
II-Meat samples
From February through December 2019; a total of 96 fresh meat samples (48 mutton and 48 beef) were obtained from Sohag city abattoir. 50 gm of each meat sample (biceps femoris) were divided into two parts; 25 gm kept frozen at – 20ºC for ten days for viability testing and 25 gm were used for detection of T. gondii by latex agglutination test (Zakaria, 2011) and microscopical examination (Liu et al., 2015).
Latex agglutination test
25 gm of each meat sample were frozen at -18 ºC for 24 hrs and thawed for obtaining meat juice (Vismara et al., 2016). Latex agglutination test were applied on meat juice using Atlas medical, UK kit following the manufacture instructions.
Microscopical examination
Positive meat samples with latex agglutination test were homogenized with 125 ml pepsin solution (2.5%) and phosphate buffer saline (PBS), then incubated with continuous shaking for 1.5 hour at 37 ºC and filtered with gauze. The homogenate was centrifuged for 10 min at 2800 rpm. Discard the supernatant and 10 ml PBS and 10 ml sodium hydroxide was added to the sediment and centrifuged again (Bayarri et al., 2010). Smear from sediment were dried on a slide, fixed and stained by Giemsa stain (Sigma Aldrish), then examined with light microscope (Olympus) at x 100 magnification.
Effect of freezing on T. gondii viability
Positive samples which interpreted as positive by latex agglutination test and microscopically confirmed tissue cyst containing samples were used for T. gondii viability after frozen for ten days at – 20 ºC. Samples were digested as mentioned before for intraperitoneal inoculation into mice (Bayarri et al., 2010). Penicillin (1,000 U/ml) and streptomycin (100 mg/ml) was added to the prepared sediment and 0.5 ml was intraperitoneal inoculated into mice groups (group for each sample); each group included three mice, two for test and one negative control mice, which were analyzed at the end of the process for each sample. Swiss Albino female mice seronegative for T. gondii were obtained from Animal laboratory Unit of Assiut University, Assiut, Egypt and maintained following the international rules in care of experimental laboratory animals. After inoculation, mice were investigated for eight weeks. Mice with acute signs as ascites, hunched appearance or emaciation were culled and the peritoneal exudates and blood were examined microscopically for tachyzoites (El-Razik et al., 2014). Serum from survived mice for eight weeks was examined by Latex agglutination test. Then mice were slaughtered and their brain fragments were squeezed and examined microscopically for T. gondii tissue cyst (Dubey et al., 2005).
RESULTS
Out of 90 serum samples of aborted women 32 (35.6%) were positive for T. gondii by ELISA; 27 represented IgG, three had IgM and two with both IgM and IgG (Table 1). T. gondii was detected by n PCR in four (12.5%) blood samples out of 32 seropositive cases; two in aborted women with IgM, one woman with IgG and one with IgM and IgG. Also; T. gondii was found in placenta tissue of 25 (78.1%) out of 32 seropositive cases; of them 24 in aborted women with IgG and one woman with both IgM and IgG and not detected in women had IgM (Table 2 and Figure 1).
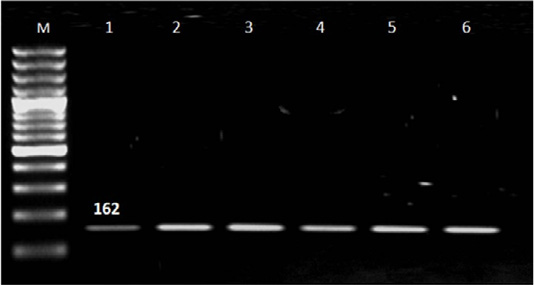
Figure 1: Results of nested PCR of T. gondii in blood and placenta tissue of seropositive aborted women. Lane M: 100 bp ladder ( Norgen Bioteck), Lane 1,2,3,4,5 and 6: Positive.
Characteristics of aborted women in Table 3 revealed that most of T. gondii seropositive cases were detected in women aged >35 years (45.5%) and aborted women live in urban communities less susceptible to infection (9.5%) than those live in rural areas (43.5%). Infection with T. gondii was reported with highest percentage in second trimester of pregnancy (43.4%). Referring to abortion history; aborted women for second (41.7%) and third (43.8%) time recorded higher infection rate than those aborted for the first time (19.2%).
Regarding factors related to T. gondii infection; living in rural area, eating insufficient cooked meat and hand washing significantly correlated with infection (P<0.01). There is no significance relation between aborted women age, trimester, abortion history, farm animal contact, contact with cat and eating raw vegetables and infection with T. gondii.
T. gondii was detected by latex agglutination test and microscopical examination in 96 mutton and beef samples (48 for each) with percentage 45.8% and 31.3%, respectively (Table 4; Figures 2 and 3). After freezing of positive meat samples for ten days at – 20°C, T. gondii lost its viability which indicated by mice inoculation and confirmed by latex agglutination test of their serum.

Figure 2: T. gondii in mutton and beef juice by Latex agglutination test. Sample 1, 4 and 6: +ve; Sample 2, 3 and 5: -ve.
DISCUSSION
Serological detection of T. gondii antibodies using ELISA in serum of aborted women was reported in Table 1, in which seropositive cases were 32 (35.6%) out of 90 serum samples. This result was lower than that reported by Mandour et al. (2017) and Matin et al. (2017). The majority of aborted women serum possesses IgG with percentage 30 referring to chronic infection in consistent with Kamal et al. (2015) and higher than that reported by Saki et al. (2015). While IgM was detected in three (3.33%) of aborted women representing acute infection lower than that mentioned by Matin et al. (2017), lastly two cases (2.22%) had both IgM and IgG. The variation of Toxoplasma antibodies level may be attributed to environmental changes, immunity level (Topley et al., 2005), cultural and food habits of communities which represented in extent of knowledge about source of infection and mode of transmission of infection. Also, hygienic practice followed in kitchen during food preparation and before eating as washing vegetables, fruits, utensils and hands. Dealing with animals especially cats and whether cat kept indoors or allowed to be outdoors. In addition; type of consumed food as meat and its products whether well, medium or improper cooked meat such as grilled mutton which considered a source of infection with T. gondii (Muflikhah et al., 2018).
Nested PCR results in Table 2 and Figure 1 revealed that the majority of IgG seropositive cases 26/27 (96.4%) represented negative result for T. gondii in blood samples while placenta tissue give positive result with 24/27 (88.9%) IgG cases and negative result in women with IgM. Presence of Toxoplasma in placenta tissue in women with IgG and absence with IgM may be related to that IgG was produced in late infection in which parasite localized in tissue and organs while in recent infection the parasite circulated in blood and not reach to tissue (Tekkesin, 2012). In addition; three (11.1%) seropositive IgG cases represented negative result with n PCR and this may be attributed to the low number of parasite in placenta tissue (Matin et al., 2017).
Referring to infection risk factors, residence, eating undercooked meat and periodical hand washing exhibited significant for T. gondii infection (P<0.01) (Table 3). Similar results were mentioned by Tammam et al. (2013) and Kamal et al. (2015). The significant association between T. gondii infection and living in rural areas may be attributed to that women in rural communities more susceptible daily to varied sources of infection as soil, farm animals and untreated water. In addition, knowledge deficiency about the disease, unhygienic practice and shortage of health care services especially in Upper Egypt (Alvarado-Esquivel et al., 2009; El-Deeb et al., 2012). Under cooked meat play an important role in disease transmission and this may be attributed to that in Egypt some traditional and commonly consumed meat meals were prepared from undercooked meat such as kebab in which mutton is the basic component, hawawshi and basterma. In addition; periodical hand washing considered a main hygienic measure in disease control and ignoring it may increase health risks. Women aged >35 years represented the highest infection rate, this may be related to increased the risk of Toxoplasma exposure with large age as in agriculture work, house activities and animals rearing (Rai et al., 1999). Regarding to habitual abortion; abortion in women for third time reported the highest present rate 43.8% which explained by reactivation or re-exposure to infection (Muna and Nadham, 1996).
Examination of T. gondii by latex agglutination test (Figure 2) and microscopical examination (Figure 3) in fresh meat samples indicated that 22 (45.8%) out of 48 mutton samples and 15 (31.3%) out of 48 beef samples were positive for T. gondii (Table 4). The high prevalence of T. gondii in slaughtered animals (sheep and cattle) exhibits a real risk of human infection following consumption of undercooked meat . Similar results mentioned by Medani and Kamil (2014) who detect T. gondii antibodies in 40.9% of sheep at Khartoum, Sudan. Lower results were reported by Zakaria (2011); Rahdar et al. (2012) and Glor et al. (2013) and higher results were obtained by Berger-Schoch et al. (2011). These differences may be attributed to geographical distribution and environmental condition as humidity, warm weather, altitude characteristics and suitable aeration, since Toxoplasma oocyst in moist condition will survive longer (Bisson et al., 2000). Also, hygienic measures followed during animal breeding and mixed breeding in which contact between different species of animals may increase the risk of infection. In addition; early disease diagnosis and veterinary care have a role in controlling of infection (Stelzer et al., 2019).
Higher infection rate was reported in sheep than in cattle. Similar results were described by Rahdar et al. (2012) and Fereig et al. (2016). This may be related to that sheep in many countries especially Egypt grassing outdoors in unhygienic condition and feeding habit of animals, as sheep feed on the ground grass which increasing its exposure to cat feces from the environment. (Sharif et al., 2006).
Freezing was used as inhibition method for parasite viability in meat tissue. Our results indicated that T. gondii losses its infectivity in mice after experimental inoculation with positive meat samples after freezing for ten days at -20 ºC. This result was similar to that reported by Kotula et al. (1991) and Alizadeh et al. (2018) who reported that storage of meat at -20 ºC for at least three days will reduce Toxoplasma contamination in meat. Efficient veterinary inspection of meat is required for providing safe and healthy food to consumers in parallel with educational health programs about T. gondii transmission, its source of infection and control measures which should be followed especially for high risk people.
Table 1: Occurrence of T. gondii antibodies in aborted women serum by ELISA.
| Method | No of examined samples | Type of samples |
T. gondii antibodies |
Total | ||||||
| IgM | IgG | IgM & IgG | ||||||||
| No | % | No | % | No | % | No | % | |||
| ELISA | 90 | Serum | 3 | 3.33 | 27 | 30 | 2 | 2.22 | 32 | 35.6 |
Table 2: Occurrence of T. gondii in blood and placenta of ELISA seropositive aborted women by nested PCR.
| ELISA | No. of examined samples | nPCR (blood samples) | n PCR (Placenta tissue) | ||||||
| +ve | % | -ve | % | +ve | % | -ve | % | ||
| IgM positive | 3 | 2 | 66.7 | 1 | 33.3 | 0 | 0 | 3 | 100 |
| IgG positive | 27 | 1 | 3.7 | 26 | 96.4 | 24 | 88.9 | 3 | 11.1 |
| IgM and IgG positive | 2 | 1 | 50 | 1 | 50 | 1 | 50 | 1 | 50 |
| Total | 32 | 4 | 12.5 | 28 | 87.5 | 25 | 78.1 | 7 | 21.9 |
Table 3: Characteristics and risk factors of T. gondii in serum samples of aborted women.
| Risk factors | No of examined samples |
Toxoplasma antibodies |
P value | |||||||
| IgM n=3 | IgG n=27 | IgM &IgG n=2 | Seropositive cases n=32 | |||||||
| No/90 | N | % | N | % | N | % | N | % | ||
| Age | 0.346 | |||||||||
| 20-25 | 22 | 1 | 4.5 | 5 | 22.7 | 1 | 4.5 | 7 | 31.8 | |
| 26-30 | 43 | 2 | 4.7 | 11 | 25.9 | 1 | 2.3 | 14 | 32.6 | |
| 31-35 | 14 | - | - | 6 | 42.9 | - | - | 6 | 42.9 | |
| >35 | 11 | - | - | 5 | 45.5 | - | - | 5 | 45.5 | |
| Residence* | 0.01 | |||||||||
| Urban | 21 | - | - | 2 | 9.5 | - | - | 2 | 9.5 | |
| Rural | 69 | 3 | 4.3 | 25 | 36.2 | 2 | 2.9 | 30 | 43.5 | |
| Farm animal contact | 0.098 | |||||||||
| Yes | 58 | 1 | 1.7 | 16 | 27.6 | - | - | 17 | 29.3 | |
| No | 32 | 2 | 6.3 | 11 | 34.4 | 2 | 6.3 | 15 | 46.9 | |
| Contact with cat | 0.191 | |||||||||
| Yes | 21 | 0 | 0 | 9 | 42.9 | 1 | 4.8 | 10 | 47.6 | |
| No | 69 | 3 | 4.3 | 18 | 26.1 | 1 | 1.4 | 22 | 31.9 | |
| Eating under cooked meat* | 0.01 | |||||||||
| Yes | 1 | 0 | 0 | 1 | 100 | 0 | 0 | 1 | 100 | |
| Sometimes | 27 | 2 | 7.4 | 17 | 63 | 1 | 3.7 | 20 | 74.1 | |
| No | 62 | 1 | 1.6 | 9 | 14.5 | 1 | 1.6 | 11 | 17.7 | |
| Eating raw vegetables | 0.907 | |||||||||
| Yes | 64 | 2 | 3.1 | 19 | 29.7 | 2 | 3.1 | 23 | 35.9 | |
| No | 26 | 1 | 3.8 | 8 | 30.8 | 0 | 0 | 9 | 34.6 | |
| Periodical hand washing * | 0.01 | |||||||||
| Yes | 31 | 0 | 0 | 4 | 12.9 | 0 | 0 | 4 | 12.9 | |
| No | 59 | 3 | 5.1 | 23 | 39 | 2 | 3.4 | 28 | 47.5 | |
| Trimester | 0.064 | |||||||||
| First | 17 | 1 | 5.9 | 2 | 11.8 | - | - | 3 | 17.6 | |
| Second | 53 | 2 | 3.8 | 19 | 35.8 | 2 | 3.8 | 23 | 43.4 | |
| Third | 20 | - | - | 6 | 30 | - | - | 6 | 30 | |
| Abortion history | 0.071 | |||||||||
|
1st time |
26 | 2 | 7.7 | 3 | 11.5 | - | - | 5 | 19.2 | |
|
2nd time |
48 | 1 | 2.1 | 19 | 39.6 | - | - | 20 | 41.7 | |
|
3rd time |
16 | - | - | 5 | 31.3 | 2 | 12.5 | 7 | 43.8 | |
* Significant factors.
Table 4: Occurrence of T. gondii in fresh meat samples.
| Positive samples | Mutton no. 48 | Beef no.48 | ||
| No | % | No | % | |
| T. gondii | 22 | 45.8 | 15 | 31.3 |
CONCLUSION
Existences of T. gondii among aborted women and in meat samples raise the importance of conducting a rapid and sensitive diagnostic method in parallel with improving public knowledge through obligated health education programs to farmers and women including disease source, mode of transmission and preventive measures. Sharing database between medical, agriculture and veterinary authorities considered a serious step in performing a strategy for early disease diagnosis, control and treatment.
Author’s contribution
NMA and AAH equally contributed in designing the study, collecting samples, literature search. AAH collecting and analysing data. NMA, AAH and MIA equally contributed in lab work, wrote and prepare the manuscript for submission.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES