Advances in Animal and Veterinary Sciences
Research Article
Derivatization of Chitin and Chitosan from Black Soldier Fly (Hermetia illucens) and Their Use as Feed Additives: An In vitro Study
Anuraga Jayanegara1*, Ratna P. Haryati2, Ainun Nafisah2, Pipih Suptijah3, Muhammad Ridla1, Erika B. Laconi1
1Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia; 2Graduate School of Nutrition and Feed Science, IPB University, Bogor 16680, Indonesia; 3Department of Aquatic Products Technology, Faculty of Fisheries and Marine Sciences, IPB University, Bogor 16680, Indonesia.
Abstract | The objective of this study was to extract chitin from black soldier fly (BSF) and to convert the substance into chitosan. These products were then used as feed additives and evaluated in an in vitro rumen fermentation system. Extraction of chitin was performed by oil removal procedure, followed by solubilization in acid and alkali solutions under elevated temperature. Conversion of chitin to chitosan was done through a deacetylation step by using NaOH. These products were evaluated in the in vitro rumen fermentation procedure according to the following treatments: control diet, consisted of a mixture between Setaria splendida grass and concentrate 60:40 w/w (CON), CON + chitin 1% (CHI1), CON + chitin 2% (CHI2), CON + chitosan 1% (CTS1) and CON + chitosan 2% (CTS2). Results showed that deacetylation degree of the extracted BSF chitin and chitosan were 33.4 and 61.6%, respectively. Chitosan addition at 1 or 2% decreased total VFA concentration as compared to control diet (P<0.05), but it was not the case for that of chitin. Chitosan addition at 2% reduced IVOMD of the diet by 9.5% (P<0.05). Chitosan addition at 2% level reduced methane emission by 9.0% as compared to control (P<0.05), but the effect was not significant when the compound added at 1%. It is concluded that chitosan derived from BSF reduces methane emission and ruminal feed degradation.
Keywords | Chitin, Chitosan, Maggot, Feed additive, Artificial rumen
Received | October 30, 2019; Accepted | January 17, 2020; Published | April 10, 2020
*Correspondence | Anuraga Jayanegara, Department of Nutrition and Feed Technology, Faculty of Animal Science, IPB University, Bogor 16680, Indonesia; Email: anuraga.jayanegara@gmail.com
Citation | Jayanegara A, Haryati RP, Nafisah A, Suptijah P, Ridla M, Laconi EB (2020). Derivatization of chitin and chitosan from black soldier fly (hermetia illucens) and their use as feed additives: an in vitro study. Adv. Anim. Vet. Sci. 8(5): 472-477.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.472.477
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Jayanegara et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Black soldier fly larvae (BSF, Hermetia illucens) has been recently considered as a promising protein source for animal feed, both for monogastrics (Cullere et al., 2016) and ruminants (Jayanegara et al., 2017a). It is characterized by its high protein content as well as good amino acid profiles (Sanchez-Muros et al., 2014). Protein itself plays an important role in the body of livestock; it serves as building blocks for various tissues and organs, and involves in the regulatory function as an integral component of various enzymes and hormones. Our previous study demonstrated that BSF contained protein in its body of more than 40% on dry matter basis (Jayanegara et al., 2017a). Other studies reported that the crude protein content of BSF ranged from 38.3 to 58.8% (Marono et al., 2015; Oonincx et al., 2015; Spranghers et al., 2017). Apart from its high protein content, BSF has other additional advantages in which it grows rapidly on various organic substrates (Spranghers et al., 2017), and does not provide any adverse effect on environment and human health.
Despite all of its potency, BSF contains considerable amount of chitin that may limit its use as animal feed. Chitin is a polymer of N-acetylglucosamine and typically present in substantial amount in insects. It had been reported that chitin content of BSF ranged from 8.7 to 9.6% (Diener et al., 2009; Kroeckel et al., 2012). Chitin is insoluble in neutral detergent solution as indicated by the high proportion of neutral detergent insoluble crude protein (NDICP) content of some insect species, including BSF (Jayanegara et al., 2017a). A negative relationship between NDICP and ruminal protein digestibility had been previously observed (Jayanegara et al., 2016). Replacement of soybean meal by BSF in a napier grass based diet resulted in a reduced ruminal ammonia concentration and organic matter digestibility (Jayanegara et al., 2017b). It has been therefore recommended to remove chitin present in BSF, at least partially, in order to enhance its nutritive value. The removed chitin, however, may provide an advantage when being used at low concentration as a feed additive since the substance has an anti-microbial property towards a broad spectrum of microbial species. Its biological activity may further be enhanced by converting it to chitosan via a deacetylation procedure.
The objective of this study was to extract chitin from BSF and to convert the substance into chitosan. These products were then used as feed additives and evaluated in an in vitro rumen fermentation system.
Materials and Methods
Chitin Extraction and Deacetylation to Chitosan
The BSF aged 25 d (pre-pupae stage) was used as the experimental material. Extraction of chitin from BSF was according to Paulino et al. (2006). Prior to chitin extraction, 1 kg of BSF sample was subjected to oil removal by using hexane for 3 h in a soxhlet extraction system. The defatted BSF was then solubilized in 1 mol/l HCl at 100oC for 60 min under a continuous stirring. Neutralization was performed by washing the solid part with aquadest. It was then added with 3 mol/l KOH and heated at 80oC for 120 min. The chitin obtained was rinsed with aquadest and dried in an oven at 80oC.
Conversion of chitin to chitosan was performed through a deacetylation procedure by using KOH. A certain amount of NaOH 40% was added to chitin extract with a ratio of 10:1 v/w, respectively. The mixture was inserted in an extractor at a temperature of 80oC for 90 min. It was subsequently filtered and inserted again in the extractor at 80oC for 24 h. Both extracted chitin and chitosan from BSF were evaluated for their deacetylation degree by using a Fourier transform infrared spectrophotometer (FTIR).
In Vitro Evaluation
An in vitro rumen fermentation procedure was conducted to evaluate the chitin and chitosan extracts from BSF. The experimental treatments were arranged as follow: control diet, consisted of a mixture between Setaria splendida grass and concentrate 60:40 w/w (CON), CON + chitin 1% (CHI1), CON + chitin 2% (CHI2), CON + chitosan 1% (CTS1) and CON + chitosan 2% (CTS2). Prior to the in vitro incubation, the grass sample was oven-dried at 60oC for 24 h and then ground by a hammer mill to pass a 1 mm screen. The sample was then mixed with the concentrate and served as the control diet. Both Setaria splendida grass and concentrate were analyzed for crude protein (CP) and ether extract (EE) contents (AOAC, 2005), neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents (Van Soest et al., 1991), and neutral detergent insoluble crude protein (NDICP) value (Licitra et al., 1996). These chemical composition determinations were conducted in duplicate.
The in vitro incubation was performed according to Theodorou et al. (1994). An amount of 0.75 g sample was incubated in a serum bottle of 150 ml capacity. A volume of 75 ml buffered rumen fluid (rumen:buffer = 1:2 v/v) was added into the bottle and flushed with CO2 in order to ensure anaerobic environment. Rumen inoculum was obtained from the rumen of two thin-tailed sheep by using a stomach tube method. The bottle was immediately closed with rubber cap and sealed with aluminium stopper. Incubation was conducted in a water bath maintained at 39oC for 24 h. Gas production was measured by using a syringe whereas methane (CH4) was determined by using a gas chromatograph (GC Shimadzu 8A, Shimadzu Corp., Kyoto, Japan) equipped with a flame ionization detector. Supernatant was separated by using a centrifuge and it was subjected to measurements of pH, ammonia (NH3) and total volatile fatty acid (VFA) as described in Jayanegara et al. (2016). In vitro dry matter digestibility (IVDMD) and in vitro organic matter digestibility (IVOMD) were determined by further incubating the residue in a pepsin-HCl solution for another 24 h at 39oC. Another set of bottles were incubated to evaluate gas production kinetics by measuring the gas at 2, 4, 6, 8, 10, 12, 24, 48 and 72 h. Kinetic parameters were obtained by fitting the gas production kinetics data to a non-linear equation proposed by Orskov and McDonald (1979). The incubation was performed in four replicates and represented by two incubation bottles per replicate.
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) according to a randomized complete block design. Different replicates or incubation runs served as the blocks. Data were checked for outlier values, i.e., values beyond –2 to 2 of their normalized residuals. These outliers were removed from the dataset. A post-hoc Duncan’s test was applied to the data for comparison among different treatment means.
Results and Discussion
The FTIR profiles of chitin and chitosan extracted from BSF are presented in Figure 1. Deacetylation procedure applied to the BSF chitin effectively removed acetyl group from the compound and increased the deacetylation degree by almost double, turned the compound to chitosan, the deacetylated product of chitin. Basal diet used in the present study (mixture of Setaria splendida grass and commercial concentrate) contained approximately 13% crude protein (Table 1). The concentrate apparently was of low quality due to its low CP content and high NDF and ADF contents.
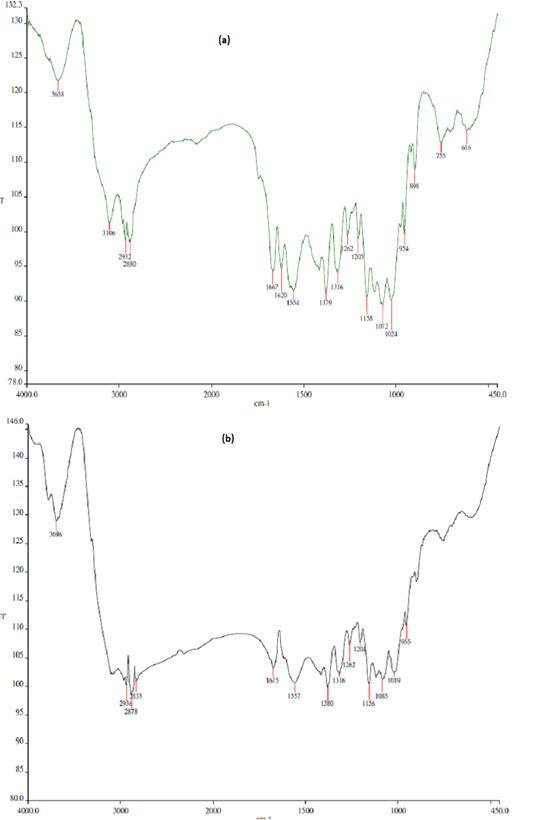
Figure 1: Fourier transform infrared (FTIR) profiles of chitin (a) and chitosan (b) derived from black soldier fly (BSF). Deacetylation degree (DD) of BSF chitin = 33.4%; DD of BSF chitosan = 61.6%.
Chitin is a natural biopolymer and it is mainly present in the exoskeletons of crustaceans and insects as well as in the cell walls of fungi. Its general properties are persistent and insoluble in water. Chitosan may be obtained from chi
Table 1: Chemical composition of Setaria splendida grass (SG), concentrate, and SG-concentrate mixture 60:40 w/w (SGC).
| Component | SG | Concentrate | SGC |
| CP (%DM) | 14.5 | 11.1 | 13.1 |
| EE (%DM) | 3.9 | 2.7 | 3.4 |
| NDF (%DM) | 71.1 | 79.9 | 74.6 |
| ADF (%DM) | 45.0 | 55.7 | 49.3 |
| NDICP (%CP) | 51.3 | 30.7 | 43.1 |
CP, crude protein; DM, dry matter; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; NDICP, neutral detergent insoluble crude protein.
tin through deacetylation procedure, typically by using a strong alkali such as NaOH or KOH (Ghormade et al., 2017). Such deacetylation increases the solubility of chitosan and its biological activity (antimicrobial property) against a wide range of microbes such as bacteria, yeast and fungi (Barbosa et al., 2019). Deacetylation degree of chitosan from BSF obtained in this study was lower as compared to some commercial chitosans. Commercial chitosans are usually extracted from crustaceans and, after deacetylation steps, have deacetylation degree of 75% or above (Goiri et al., 2009). Different chitosan sources, characteristics, deacetylation procedures and analytical methods may result in such variation of deacetylation degree. Solubility of chitosan apparently also determines its deacetylation degree. Belanche et al. (2016a) reported that insoluble and soluble chitosan had deacetylation degree of 80% and >85%, respectively. Further, it was characterized that insoluble chitosan had viscosity of 50 mPa·s, whereas soluble chitosan had viscosity of 140 mPa·s in 10 ml/l acetic acid solution at 25oC.
Addition of BSF chitin or chitosan did not alter ruminal pH and ammonia concentration in the in vitro system (Table 2). Chitosan addition at 1 or 2% decreased total VFA concentration as compared to control diet (P<0.05), but it was not the case for that of chitin. Chitosan addition at 2% reduced IVOMD of the diet by 9.5% (P<0.05), whereas other treatments were similar to control. Addition of 1% chitin increased gas production at 24 h in comparison to control (P<0.05; Table 3). On the contrary, addition of 2% chitosan decreased the gas production than that of control (P<0.05). Gas production potential (b) and its production rate (c) were not altered by addition of either chitin or chitosan. Chitosan addition at 2% level reduced methane emission by 9.0% as compared to control (P<0.05), but the effect was not significant when the compound added at 1%.
Methane reduction effect of chitosan is apparently related to its antimicrobial property on a wide variety of microbes including methanogens, the primary microbial group in
Table 2: In vitro ruminal fermentation parameters of chitin or chitosan addition from black soldier fly.
| Treatment | pH |
VFA (mmol/l) |
NH3 (mmol/l) |
IVDMD (%) |
IVOMD (%) |
| CON | 7.10 |
132b |
7.52 | 51.2 |
54.8b |
| CHI1 | 7.07 |
131b |
7.25 | 50.4 |
54.8b |
| CHI2 | 7.08 |
118ab |
8.44 | 51.5 |
54.6b |
| CTS1 | 7.00 |
112a |
8.25 | 50.0 |
53.3b |
| CTS2 | 7.00 |
106a |
7.04 | 47.4 |
49.6a |
| SEM | 0.020 | 4.28 | 0.298 | 1.31 | 1.41 |
| P-value | 0.100 | 0.011 | 0.323 | 0.075 |
0.029 |
Different superscripts in the same solumn are significantly different at P<0.05.
CON, Setaria splendida grass and concentrate mixture 60:40 w/w; CHI1, CON + chitin 1%; CHI2, CON + chitin 2%; CTS1, CON + chitosan 1%; CTS2, CON + chitosan 2%.
VFA, volatile fatty acid; NH3, ammonia; IVOMD, in vitro organic matter digestibility; SEM, standard error of mean.
Table 3: Gas production and methane (CH4) emission of chitin or chitosan addition from black soldier fly.
| Treatment |
Gas24 (ml) |
b (ml) |
c (/h) |
CH4 (µl) |
| CON |
43.6b |
99.8 | 0.024 |
200bc |
| CHI1 |
47.4c |
99.0 | 0.025 |
208c |
| CHI2 |
41.1ab |
98.6 | 0.022 |
188ab |
| CTS1 |
41.3ab |
100.2 | 0.022 |
188ab |
| CTS2 |
39.9a |
98.9 | 0.022 |
182a |
| SEM | 0.752 | 2.79 | 0.001 | 2.94 |
| P-value | 0.001 | 0.970 | 0.123 |
0.006 |
Different superscripts in the same solumn are significantly different at P<0.05.
CON, Setaria splendida grass and concentrate mixture 60:40 w/w; CHI1, CON + chitin 1%; CHI2, CON + chitin 2%; CTS1, CON + chitosan 1%; CTS2, CON + chitosan 2%.
Gas24, gas production at 24 h; b, gas production potential; c, gas production rate.
the rumen responsible for methanogenesis from various susbtrates. It had been demonstrated that addition of 5% chitosan reduced methanogens population from 3.27 to 1.78 × 103 × ∆CT, and methane emission was reduced by approximately 43%, i.e., from 5.24 to 2.99 mmol/d (Belanche et al., 2016b). The magnitude of methane decrease in the current study was comparatively lower in comparison to that of Belanche et al. (2016b). This could be attributed to the lower concentration of chitosan used in the present study, i.e., 2% against 5% in that study. In addition, deacetylation degree of BSF chitosan here was lower, i.e., slightly above 60%, whereas Belanche et al. (2016b) used a chitosan with >85% deacetylation degree. Lower protozoa population by addition of chitosan may also contribute to the lower methane emission. It was shown that chitosan had an antiprotozoal effect (Belanche et al., 2016a). Part of the methanogens live symbiotically with protozoa as they obtain hydrogen from the fauna to synthesis methane (Morgavi et al., 2012). Decreasing protozoa population may therefore lead to a reduced methanogens population and the methanogenesis.
Lower IVOMD, total VFA and gas production at 24 h by addition of 2% chitosan may indicate its negative effect on feed degradation in the rumen. Apart from its possible antimicrobial effect on rumen microbes, chitosan may also interact with feed nutrients and partially prevent their ruminal degradation. A decrease in ruminal feed degradation contributes to a lower methanogenesis as well since the degradation produces hydrogen as a main substrate for the reaction (Ungerfeld, 2018). Despite of this fact, lower ruminal feed degradation does not always lead to a lower animal performance and productivity. When a high quality protein is less degraded in the rumen and, as a consequence of it, generates more by-pass protein, the overall metabolizable protein supply for ruminant livestock may be enhanced. It has been well established that metabolizable protein supply is positively correlated with animal performance (Owens et al., 2014). Therefore, the mechanism of action of chitosan in decelerating nutrient degradation in the rumen is to a certain extent similar to tannin by forming complexes with feed nutrient particularly protein (Kondo et al., 2014; Jayanegara et al., 2019).
It had been proposed that chitosan mode of action as a natural antimicrobial compound is by changing cell permeability and promotes hydrolysis of cell wall (peptidoglycan component) of the microbes (Kong et al., 2010). Such peptidoglycan layer is more prominent in Gram-positive bacteria (mostly cellulolytic bacteria) than in Gram-negative bacteria (mostly amylolytic bacteria). It is therefore unsurprising that cellulolytic bacteria is more negatively affected in the presence of chitosan as compared to amylolytic bacteria. It had been repeatedly demonstrated that chitosan addition reduced molar proportion of acetate while increased molar proportion of propionate (Goiri et al., 2009; Belanche et al., 2016b). Acetate is a main fermentation product of cellulolytic bacteria whereas propionate is a main fermentation product of amylolytic bacteria in the rumen. The shift of VFA composition towards more propionate and less acetate contributes to the lower methanogenesis since, stoichiometrically, propionate production consumes hydrogen while acetate production releases hydrogen (Alemu et al., 2011).
Conclusion
Chitosan derived from BSF reduces methane emission and ruminal feed degradation, especially when added at 2% DM. However, its original chitin has negligible effects on rumen fermentation parameters as measured in vitro.
ACKNOWLEDGEMENT
The authors are grateful to Kementerian Riset, Teknologi dan Pendidikan Tinggi (KEMENRISTEKDIKTI) for funding this research through “Penelitian Dasar Unggulan Perguruan Tinggi - PDUPT” grant, year 2019, contract number 4200/IT3.L1/PN/2019.
CONFLICT OF INTEREST
All authors declare that there is no conflict of interest.
authors contribution
AJ designed and supervised the experiment, performed the statistical analysis and wrote the manuscript; RPH and AN carried out the experiment; PS, MR and EBL supervised the experiment.
References






