Advances in Animal and Veterinary Sciences
Research Article
Does Bougainvillea spectabilis protect Swiss Albino Mice from Aflatoxin-induced Hepatotoxicity?
Nidhi Mishra1, Vijay Lakshmi Tandon1, Kuldeep Dhama2, Rekha Khandia3, Ashok Munjal3*
1Department of Bioscience and Biotechnology, Banasthali Vidyapith, Banasthali-304 022 (Rajasthan); 2Division of Pathology, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly-243122 (Uttar Pradesh); 3Department of Genetics, Barkatullah University, Bhopal-462026 (M.P.), India.
Abstract | We have investigated the protective effect of oral administration of the ethanolic extract of Bougainvillea spectabilis leaves against aflatoxin B1 (AFB1) induced hepatic injuries among male Swiss albino mice. AFB1 exposure has significantly increased (P< 0.05) the lipid peroxidase activity and decreased the activities of various antioxidant enzymes viz. superoxide dismutase (57.58%), catalase (24.14%), glutathione peroxidase (44.21%), glutathione S-transferase (44.07%) and glutathione reductase (13.51%). It also declined the level of ascorbic acid, reduced glutathione and protein contents by 47%, 34% and 24 % respectively. It markedly decreased lipid peroxidation (TBARS level) with concomitant stimulation (P < 0.05) of antioxidants (enzymatic and non – enzymatic both). HPTLC analysis revealed availability of sufficient amount of flavonoids, alkaloids and phenolic compounds in the extract of B. spectabilis leaves might be responsible for the improved efficiency of antioxidant system and subsequent protection from aflatoxicity.
Keywords | Aflatoxin, Hepatoprotective, Antioxidant, Bougainvillea spectabilis, Swiss albino mice
Editor | Yashpal S. Malik, Indian Veterinary Research Institute (IVRI), Izatnagar 243122, Bareilly, Uttar Pradesh, India.
Received | May 10, 2016; Accepted | May 28, 2016; Published | May 29, 2016
*Correspondence | Ashok Munjal, Department of Genetics, Barkatullah University, Bhopal-462 026 (M.P.), India; Email: [email protected]
Citation | Mishra N, TandonnVL, Dhama K, Khandia R, Munjal A (2016). Does Bougainvillea spectabilis protect swiss albino mice from aflatoxin-induced hepatotoxicity? Adv. Anim. Vet. Sci. 4(5): 250-257.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.5.250.257
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Mishra et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Alflatoxins are secondary metabolites produced by several strains of filamentous fungi mostly by Aspergillus flavus and A. parasiticus. These are closely related to di-furanocoumarin compounds (Steyn, 1995; Williams et al., 2004). Epidemiological studies suggest that elevated dietary exposure of aflatoxin can induce liver cancer in humans (Van Rensburg et al., 1985; Liu et al., 2013; Hamid et al., 2013; Bhakuni et al., 2016). However, Angusbhakorn et al. (1990) have reported that even a single dose of aflatoxin is sufficient to induce liver tumours among rats.
The International Agency for Research on Cancer (IARC) (1993) has classified aflatoxin as a highly potential carcinogenic agent (Class I). Later on several strategies have been evolved for reducing the carcinogenic effect of aflatoxins. These strategies include degradation, destruction, inactivation or removal of mycotoxins through chemical and physical methods. However, the application of these strategies on food stuffs resulted in alteration of organoleptic characteristics and nutritional values of food (Ellis et al., 1991; Cazzaniga et al., 2001). Moreover, antimutagenic agents have also been suggested to inhibit the genotoxic effects of AFB1 (Madrigal-Bujaidar et al., 2015), but results were not optimal and had certain side effects. Therefore, researchers switched to natural products. Plant derived natural products are proven to have potential of curing cancer with minimal side effect and economical.
Plant-based products are rich source of antioxidants and secondary metabolites and therefore effectively control several ailments, like oxidative stress (Sen, 1995). Previous studies using Thonningia sanguinea (Gyamfi and Aniya, 1998) Phyllanthus amarus (Naaz et al., 2007), Zizyphus spina-christi (Abdel-Wahhab et al., 2007), Jatropha curcas (Balaji et al., 2009) and black tea (Jha et al., 2011) have revealed hepatoprotective effects against AFB1 induced hepatotoxicity.
B. spectabilis (Family Nyctaginaceae) is the common evergreen ornamental woody plant inhabiting in tropical regions. The leaves of B. spectabilis possesses several medicinal properties viz. antiviral (Narwal et al., 2001), insecticidal activity (Thangam and Kathiresan, 1990), anti-diabetic (Narayanan et al., 1987; Bates et al., 2000), anti-inflammatory (Mandal et al., 2015), anti-oxidative potential (Medpilwar et al., 2015) and cytotoxicity against cancer cell lines (Do et al., 2016) etc. Besides several known medicinal properties of B. spectabilis leaves, its experimental validation of hepatoprotective nature is still to be studied. The present investigations aimed to elucidate the hepatoprotective effect of ethanolic extract of B. spectabilis against AFB1 induced hepatotoxicity.
Materials and Methods
Test Animals and their Rearing
Adult male and female Swiss albino mice procured from CCS Haryana Agricultural University, Hisar were mated and resulting progeny was maintained in a well ventilated animal house with 12:12 light / dark cycle. Only male mice (60 days old and 30±5 g BW) were used for the experimental purpose, due to its higher susceptibility to aflatoxin (John and Paul, 1994).
Test animals were kept in polyproline cages with iron bar tops and maintained on standard pellet diet (Hindustan Levers). Tap water was made available ad libitum. As far as possible, necessary sterile conditions were provided and cleanliness was maintained in the animal cages as well as in the room. Prior approval for experiments was taken from Institutional Animal Ethics Committee as per CPCSEA (Govt. of India) norms.
Preparation of the AFB1 Solution and Extraction of B. spectabilis Ethanolic Extract (Hereafter will be written as EEBs)
Crystalline AFB1 (from Aspergillus flavus), purchased from Sigma, was dissolved in dimethylsulfoxide (DMSO) and further diluted with distilled water to the required concentration. The final solution of AFB1 contained 1% dimethyl sulfoxide. While for the extraction of ethanolic extract of B. spectabilis the leaves of B. spectabilis collected from Banasthali University campus were oven dried and powdered. The powdered material was extracted using ethanol by soxhlet method. It was further dried at 40±1˚C, which was used for the experimentation.
Division and Treatment of Animal Groups
Male Swiss albino mice were divided randomly into six groups having six mice each and were treated orally for 30 days. Group I, Control (Normal saline, 0.9%); Group II, AFB1 (2µg/30 g body weight); Group III, EEBs (400mg/kg, orally); Group IV, EEBs (800mg/kg, orally); Group V, AFB1 + EEBs (400mg/kg, orally); Group VI, AFB1 + EEBs (800mg/kg, orally) after 2 hrs.
Preparation of Post Mitochondrial Supernatant (PMS)
Tissue homogenate of liver was prepared following Mohandas et al. (1984) method. The dissected liver was homogenized in pre-chilled motar and pestle with phosphate buffer (0.1M, pH 7.4). The homogenate was centrifuged at 800 g for 5 min at 4˚C to separate the nuclear debris. The supernatant was again centrifuged at 1200 g for 30 min at 4˚C to get a clear homogenate (PMS).
Biochemical Analysis
The supernatant obtained was used for assay of total protein (Lowry et al., 1951) using Folin reagent and Bovine serum albumin (BSA) as standard, ascorbic acid content was determined using the method described by Majhi et al. (2000). The basis of ascorbic acid content method is 2, 4 dinitrophenyl hydrazine reagents incubated at 60˚C for 1 hr. Absorbance was taken at 540 nm and the results were expressed as ascorbic acid/ 100g tissue wet weight. The GSH content was determined using the method of Jollow et al. (1974). The base of GSH determination is the reaction of Ellman’s reagent 5, 5-dithiobis (2-nitrobenzoic acid) (DTNB) with the thiol group of GSH at pH 8.0 to give yellow color of 5-thiol-2-nitrobenzoate anion. Cytoprotective enzymes, GPx activity was measured following Mohandas et al. (1984). Its activity depends on oxidation of NADPH and recorded at 340 nm. The GPx activity was calculated as nmols NADPH oxidized min−1 mg−1 protein, using a molar extinction coefficient of 6.22×103M−1 cm−1, GST activity by Habig et al. (1974) method. GST activity was recorded at 340 nm and its activity calculated as nmols CDNB conjugates formed min−1 mg−1 protein using a molar extinction coefficient of 9.6×103M−1 cm−1, GR activity was determined following Dobler and Anderson (1981), Superoxide dismutase activity was measured by the method of Dhindsa et al. (1981). The absorbance was recorded at 560 nm. Results obtained due to the formation of Formosan, a reaction product of NBT, Catalase activity was measured by method of Claiborne (1985). CAT activity was determined by measuring the exponential disappearance of H2O2 absorbance and it was recorded at 240 nm. Catalase activity was calculated in terms of H2O2 consumed min-1mg-1protein. TBARS content in liver homogenate was determined by method as given by Utley et al. (1967). The ratio of LPO was expressed as nmols of thiobarbituric acid reactive substance (TBARS) formed h−1 g−1 tissue using molar extinction coefficient of 1.56×105M−1 cm−1. All the enzymatic assays were performed at particular nm using spectrophotometer.
Table 1: Effect of co-administration of B. spectabilis extract on AFB1 induced alterations in some cytoprotective enzymes among Swiss albino mice
|
Treatments (meana ± S.E.M.) |
||||||
|
Parameters |
Control |
AFB1 |
EEBs (400mg/kg) |
EEBs (800mg/kg) |
AFB1 +EEBs (400mg/kg) |
AFB1 + EEBs (800mg/kg) |
|
LPO (nmols TBARS h-1g1 tissue) |
8.12±0.03 |
22.67±0..02b |
7.81±0.02 |
9.56±.04 |
17.42±0.16 c |
14.06±0.01 c |
|
SOD(Unit mg -1 Protein h -1) |
12.99±0.17 |
5.51±0.12b |
13.43±0.09 |
12.70±.15 |
7.27±0.15 c |
7.54±0.12 c |
|
CAT(µmols H2O2 consumed Min -1 mg -1 protein) |
216.24±1.98 |
164.03±1.04b |
215.72±1.60 |
224.18±1.58 |
171.98±2.15 c |
180.99±2.02 c |
|
GST(nmoles CDNB conjugates formed min-1 mg-1 protein) |
191.70±13.54 |
107.20±1.70b |
189.70±3.26 |
185.65±2.24 |
129.50±2.09 c |
136.26±3.59 c |
|
GPx (µg of glutathione utilized min -1 mg -1 protein) |
50.06±5.57 |
27.93±3.20b |
52.57±2.90 |
52.23±3.20 |
34.91±1.91 c |
38.33±1.94 c |
|
GR (µmol NADPH oxidized min -1 mg -1 protein) |
145.50±2.29 |
125.83±1.97b |
148.79±1.12 |
146.85±1.14 |
130.96±1.14 c |
133.81±1.27 c |
aMean values of six reading; bData are significant at P<0.05 as compared to control; cData are significant at P<0.05 as compared to aflatoxin treated groups
Histopathological Studies
Liver of test organism was fixed in 10% formalin. Liver tissue was washed thoroughly in slow running tap water for 2-3hrs. Then, the washed tissue was dehydrated in descending grade of isopropanol and finally cleared in xylene. The tissue was embedded in paraffin wax. Sections were taken at 5 µm thicknesses, stained with haematoxylin and eosin. These were observed under microscope at 400x.
High Performance Thin Layer Chromatography (HPTLC)
B. spectabilis leaves samples were prepared in methanol. For characterization of phytoconstituents, aluminium silica gel 60F254 (Merck # 5564) was used with mobile phase chloroform and methanol in the ratio of 9:1. Fifteen microliter leaves sample was applied to the plate and plate was developed in a Camag twin trough chamber to a distance of 8 cm. Chamber was previously equilibrated with the mobile phase for 45 min. The developed HPTLC plate was dried using dryer for 2 min. It was scanned at 366 nm (λmax) for quantification using Camag scanner having deuterium lamp.
Statistical Analysis
Results are expressed as mean ± standard error (S.E.M). Statistical significance between the different groups was determined by one way Analysis of Variance (ANOVA) using the SPSS (Ver. 16). Post hoc testing was performed for inter-group comparisons using the Tukey multiple comparison test at P<0.05. Whenever, sphericity was significant, degree of freedom and F-value are corrected by Huynh Feldt epsilon.
Results and Discussion
Biochemical analysis of the liver revealed that AFB1 has induced significant increase (P<0.05) in lipid peroxidase (LPO) (Table 1). Increased content of TBARS is a well-known feature of AFB1 toxicity. The increased TBARS could be attributed to the reduction in detoxifying hyperperoxides in AFB1 induced hepatocellular carcinoma (HCC). It is an autocatalytic free radical process and could be responsible for DNA damage (Shirali et al., 1994; Mustafa et al., 2015) which results in impaired membrane function, structural integrity (Gutteridge and Halliwell, 1988) and inactivation of number of membrane bound enzymes and protein receptor. Inclined level of TBARS content in AFB1 treated rat and mice liver tissue has also been previously reported (Verma and Nair, 2002; El-Gibaly et al., 2003).
Lipid peroxidation could be naturally protected by antioxidant enzymes and SOD, CAT and GPx since these acts as important scavangers of superoxide ion and hydrogen peroxide. Significant reduction in the activities of various antioxidant enzymes (superoxide dismutase (57.58%), catalase (24.14%), glutathione peroxidase (44.21%), glutathione S-transferase (44.07%) and glutathione reductase (13.51%) among Group II mice (Table 1) in comparison to control (Group I; received normal saline) mice was observed. This finding is negatively correlated with TBAR content and supporting the earlier reports (Choudhary and Verma, 2005). However, the oral administration of AFB1 and EEBs (Group V and VI) had significantly increased the level approximately to normal not only in TBAR content but also the activities of antioxidant enzyme (Table 1).
Aflatoxin AFB1 is a powerful carcinogen for human and many animal species, including rodents (Fetaiha et al., 2014), non-human primates and fish (Kimura et al., 2004; Santacroce et al., 2008). It can increase the rate of DNA adducts, histidine revertants, chromosomal aberration, micronucleus and sister chromatid exchange (Anwar et al., 1994; Witt et al., 2000).
Declined level of Mn-SOD in tumor cells has been reported (Oberely and Buttener, 1979) in AFB1 induced toxicity in rats’ liver after 10 days exposure to aflatoxin. Increased lipid peroxides level is associated with decreased activity of SOD, as SOD inhibits hydrogen peroxides. Same way decreased level of GSH in AFB1 treated animals was observed which may be due to its utilization by excessive amount of free radical generated by lipid peroxidation in cancerous cells. Concentration of Liver GPx, CAT and GR were also decreased in AFB1 intoxicated animals, might be due to continuous overload of ROS particularly of hydrogen peroxide produced from catalytic reaction of superoxide by SOD in the cancerous cells of liver. The GPx perform its activity in cytosolic fraction whereas Catalase is performing its activity in microbodies. These results are comparative with the results of Meki et al. (2004), who reported significant reduction in GPx and GR activities in liver after 10 days of AFB1 treatment.
Likewise, the non-enzymatic antioxidants viz., ascorbic acid and reduced glutathione as well protein content was also markedly declined in aflatoxin treated group II in comparison to control group I (Figure 1). Level of Vitamin C in AFB1 intoxicated animals was also decreased which may be due to the utilization of Vitamin C for scavenging the ROS produced by the cancerous cells and AFB1 metabolites. The reduced Vitamin C in aflatoxin carcinoma is also reported by Premalatha and Sachdanandam (1999). But the administrations of EEBs doses along with aflatoxin are succeeded to some extend in restoring all biochemical parameters towards normal values.
Glutathione plays a critical role in cellular functions, which includes maintenance of thiol status of protein, the destruction of H2O2, lipid peroxides, free radicals, translocation of amino acids across cell membrane, the detoxification of drugs (James and Harbison, 1982). Liver GR is an enzyme responsible for the conversion of Glutathione disulphide (GSSR) back to reduced glutathione. GSSR is formed during the detoxification of H2O2 by GPx. GSH is component of three detoxifying enzyme namely GPx, GR and GST. It is also taking part in detoxification of carcinogen or its metabolites by conjugation. Reduced GR in AFB1 treated group confirmed the higher production of GSSR due to the higher rate of detoxification of H2O2 by GPx.
The treated animals with EEBs have demonstrated a significant increase in enzymatic antioxidant (P<0.05) and decrease in lipid peroxides levels as compare to aflatoxin treated groups. This observation leads to the inference that the B. spectabilis treatment along with aflatoxin counteract the abnormal increase in lipid peroxidation induced by aflatoxin and alleviated the harmful effect.
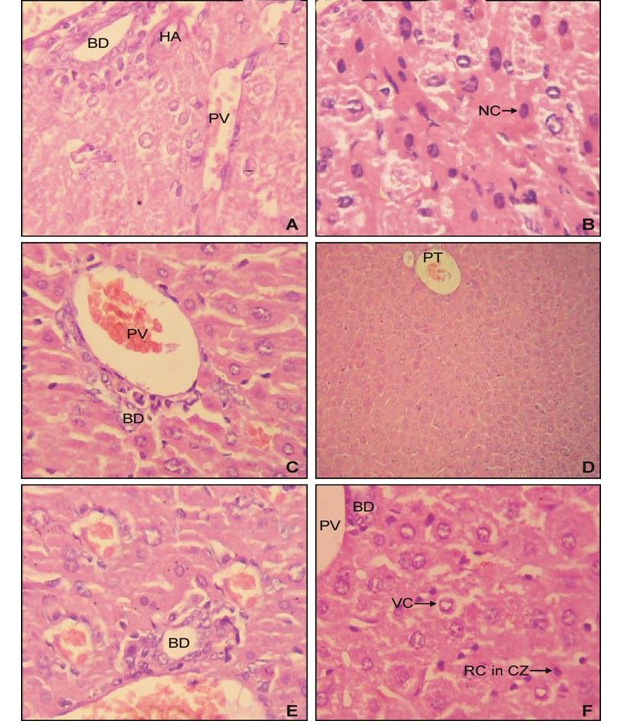
Histopathological analysis of liver samples of group II since revealed the prominent periportal necrosis (Figure 2B) in comparision to normal liver of control (Figure 2A). The group IV mice simultaneously treated with AFB1 and EEBs (400mg/kg BW) revealed vacuolar degeneration of
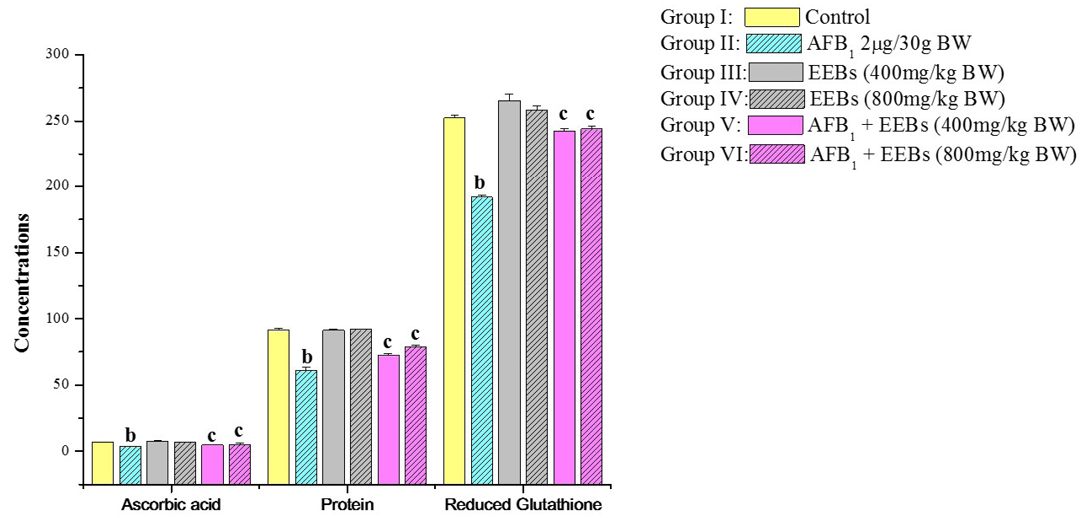
Figure 1: Effect of EEBs on ascorbic acid (ascorbic acid/100g tissue wet weight), protein (mg g-1 fresh wt) and reduced glutathione (nmols GSH g-1 tissue) in AFB1 intoxicated rats
a) Mean values of six reading; b) Data are significant at P<0.05 as compared to control; c) Data are significant at P<0.05 as compared to aflatoxin treated groups
periportal hepatocytes however necrotic effect has been diminished (Figure 2D). Whereas normal hepatic parenchyma has been observed among group VI (Figure 2E) demonstrated the potential effect of B. spectabilis extract to heal liver injuries induced by AFB1 (Figure 2C, 2D and 2E).
A) from control group I mice; B) from group II showing the effect of AFB1 which had induced prominent periportal choronic necrosis; C) from group III revealed normal architecture; D) from group V showing the hepatoprotective effect of and EEBs (400 mg/kg BW) having vacuolar degeneration but no necrosis; E) from group V with normal histopathology; F) from group VI revealing the hepatoprotective effect of and EEBs (800 mg/kg BW) against AFB1showing normal hepatic parenchyma
HPTLC analysis of EEBs depicts eight spots corresponding to the Rf 0.14, 0.18, 0.21, 0.24, 0.28, 0.33, 0.56 and 0.93 respectively (Figure 3) indicating the chief phyto-constituents of this plant are flavonoids, alkaloid and phenolic acid. Flavonoids were identified on the basis of previous reports include apigenin 7-o-β-glucopyranoside at Rf 0.18 (Modnicki et al., 2007), rutin at 0.33 (Janbaz et al., 2002) and quercetin at Rf 0.93 (Kumar et al., 2010). Rf values 0.14 (Petasinine; Bai et al., 2006), 0.21 (Jr Ruiz et al., 1977) and 0.28 (macrocyclic alkaloids budmunchiamines; Rajkumar and Sinha, 2010) were corresponding to alkaloids, whereas 0.24 (gallic acid; Rakesh et al., 2009) and 0.59 (chlorogenic acid; Bilusic et al., 2005) belongs to phenolic acids.
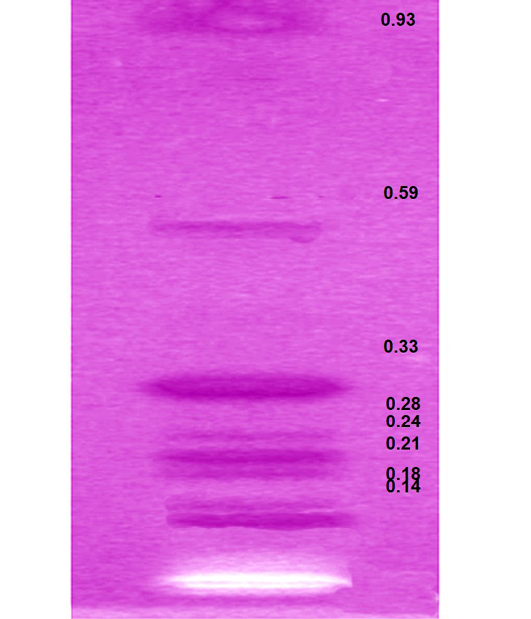
Figure 3: HPTLC plate showing the developed bands of ethanolic extract of B. spectabilis and their respective Rf values
Gallic acid acts as an antioxidant and helps to protect against oxidative damage. Phenolic compounds also act as reducing agents and are capable of inhibiting, quenching free radicals to terminate the radical chain reaction (Shukla et al., 2009). The hepatoprotective effect of different flavinoids has already been reported for rutin (Janbaz et al., 2002) and quercetin (Janbaz et al., 2004) from natural sources and various fruits (Madrigal-Santillán et al., 2014). Francis et al. (1989) and Caius (1986) have reported the potency of flavonoids to inhibit the action of AFB1-DNA adduct formation and it express its ability to suppress the development of AFB1-induced neoplasia susceptible species. Hence, it can be accomplished that the hepatoprotective effects of EEBs could be attributed to its various phyto-constituents and might be responsible for suppressing the toxicity initiated by Aflatoxin B1. Alkaloids were also known for its anti-tumour property (Corvalan et al., 1988; Jiang et al., 2016).
From the experimental findings, it can be inferred that concurrent administration of AFB1 along with EEBs has significantly reversed the altered levels of LPO and antioxidant enzymes. Biochemical analysis and histological investigation suggests that EEBs (800mg/kg BW) seems to be more effective in removal of LPO accumulated as a result of AFB1 toxicity as well as reverting the effects of prominent peripotal choronic necrosis to the normal hepatic parenchyma strongly suggests its possibility of chemopreventive potential.
Acknowledgments
Authors of the manuscript thank and acknowledge their respective Universities/ Institutes.
Conflicts of Interest
The authors declare that no conflict of interest.
Authors’ contribution
Nidhi Mishra executed the experimental work, Vijay Laxmi Tandom and Ashok Munjal designed the study. Ashok Munjal and Rekha Khandia interpreted the results and written the manuscript, Kuldeep Dhama reviewed the manuscript.
References