The Journal of Advances in Parasitology
Research Article
In vitroAntioxidant and Anticoccidial Studies of Pentaclethra macrophylla Extracts Against Eimeria magna, Eimeria flavescens, Eimeria intestinalis Eimeria stiedaeOocysts and Sporozoites of Rabbits
Yamssi Cedric1*, Vincent Khan Payne1, Noumedem Anangmo Christelle Nadia1, Norbert Kodjio2, Etung Kollins1, Leonelle Megwi1, Jules-Roger Kuiate2
1Research Unit of Biology and Applied Ecology, Faculty of Science University of Dschang, Cameroon; 2Research Unit of Microbiology and Antimicrobial Substances, Faculty of Science, University of Dschang, Cameroon.
Abstract | Background and Objective: One of the most devastating and discouraging constraints to rabbit production is coccidiosis. Thus, coccidiosis is probably the most expensive and wide spread infectious disease in commercial rabbit systems. This study was therefore carried out to validate the use of Pentaclethra macrophylla (P.M) in fighting against coccidiosis. Materials and Methods: The dried stem bark of P. macrophylla was pulverized using an electrical grinder under hygienic conditions. Four types of extracts (methanol, hexane, ethyl acetate, and infusion extracts) were prepared to compare their anticoccidial and antioxidant activities. Sporulation inhibition bioassay was used to evaluate in vitro anticoccidial activity of P. macrophylla extracts on sporulation of Eimeria magna, Eimeria flavescens, Eimeria stiedae and Eimeria intestinalis oocysts and sporozoites. In this assay, Petri dishes of 5 ml containing 1000 unsporulated oocysts per milliliter were exposed to five concentrations of extracts in 2.5% Potassium dichromate solution (2.5, 5, 10, 20 and 30 mg/ml) for oocysticidal activities and 125, 250, 500, 750 and 1000 μg/ml for in vitro anti-sporozoidal activities. The set up was examined after 24 and 48 hours for oocysticidal activities and after 12 and 24 hours for anti-sporozoidal activities. The in vitro antioxidant activity was determined by measuring the ferric reducing-antioxidant power (FRAP), the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and nitric oxide (NO) radical scavenging. Total flavonoids and total phenolic contents were also evaluated. Cytotoxicity of the methanol extract was determined against animal cell line fibroblast L929 cells using MTT assay. The impact of toxicity was established by analysing Selectivity Index values. To justify these activities, phytochemical screening was made. Results: The highest oocysticidal efficacy was 72.00±1.00 % at 30 mg/ml of methanolic extract against Eimeria intestinalis after 48 hours of incubation. The lowest efficacy was 7.00±4.36% (E. flavescens) at 2.5 mg/ml of the infusion extract after 48 hours of incubation. For each concentration and for all the Eimeria species, the methanol extracts were more efficient. The general tendency was that of a decrease in inhibition rate with an increase in incubation time. The highest viability inhibitory percentage was 80.33 at 1000 μg/ml of P. macrophylla methanolic extract against E. intestinalis sporozoites. The in vitro antioxidant activity of P. macrophylla extracts showed that they possess antioxidant activities against DPPH• and NO• radicals and iron reducing power. The best antioxidant activity were observed with the methanolic extract on the DPPH• radical (IC50 6.32 µg/ml), nitric oxide radical (79.54%) compounds and iron reducing power value (2.50). The cytotoxicity of the most active extract (Methanolic extract) exhibited CC50 of >30 µg ml against fibroblast L929 cell lines, suggesting that the compound was not toxic. Phytochemical screening showed the presence of alkaloids, flavonoids, saponins and phenols. Conclusion: Our findings, have scientifically validated the use of P. macrophylla in fighting against Coccidiosis.
Keywords | Pentaclethra macrophylla, Anticoccidial, Antioxidant, Eimeria species
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 06, 2018; Accepted | August 30, 2018; Published | September 28, 2018
*Correspondence | Yamssi Cedric, Research Unit of Biology and Applied Ecology, Faculty of Science University of Dschang, Cameroon; Email: [email protected]
Citation | Cedric Y, Payne VK, Nadia NAC, Kodjio N, Kollins E, Megwi L, Kuiate JR (2018). In vitro antioxidant and anticoccidial studies of pentaclethra macrophylla extracts against eimeria magna, eimeria flavescens, eimeria intestinalis eimeria stiedae oocysts and sporozoites of rabbits. J. Adv. Parasitol. 5(3): 38-48.
DOI | http://dx.doi.org/10.17582/journal.jap/2018/5.3.38.48
Copyright © 2018 Cedric et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
With the increase in human population especially in a developing country like Cameroon, the supply of enough animal protein from the five principal livestock species (cattle, sheep, goats, swine and poultry) had become difficult, hence the interest in micro livestock such as rabbit because its production has enormous potential in alleviating the problem of animal protein supply in developing countries. Rabbit meat is one of the white meat which is highly consumed by people because of it’s low cholesterol and fat content and high levels of essential amino-acid (Al-Mathal., 2008). The important attributes of rabbits as micro livestock include small body size, short generation interval, ability to utilize less competitive feeds, rapid growth, potentials for genetic improvement and production of high quality meat and useful by-products (Olowofeso et al., 2012).
Even with the increasing interest in rabbit production, the rabbit industry is faced with several challenges. One of the most devastating and discouraging constraints to rabbit production is coccidiosis. Coccidioses constitute a major parasitic disease in poultry and other domestic animals, including rabbits (Yamssi et al., 2018). Eleven distinct Eimeria species have been identified in rabbits (Oryctolagus cuniculus), with 10 species colonizing the intestinal tract and one species (E. stiedae) infecting the biliary ducts of the liver (Al-Mathal, 2008). Most of these Eimeria species affect rabbit production and, according to their level of pathogenicity can cause reduced growth rate, feed conversion and increased mortality. Thus, coccidiosis is probably the most expensive and wide spread infectious disease in commercial rabbit systems.
Indeed, most of the current anti-coccidial drugs show low efficacy and cause deleterious side effects (Hamad et al., 2014). The extensive use of chemical anti-coccidial drugs in controlling this disease has led to the development of drug-resistant parasites (Ebtesam and Al-Mathal, 2010) and residual effects of drugs in meat of animals. Scientists all over the world are shifting towards alternative approaches for the control of parasitic problems (Hamad et al., 2014).
There is a close relationship between oxidative stress and coccidioses. According to Yamssi et al. (2017, 2018),when a healthy animal ingest sporulated oocysts these parasites go to the intestine or liver (in the case of hepatic coccidiosis) and causes inflamations. The inflammation process leads to the over production of free radicals. These free radicals are not only toxic to the parasites they are equally toxic to the host by causing lipids peroxidations, damaging DNA and proteins. Today, the use of antioxidants as anticoccidial remedies, therefore holds promise as an alternative in the control of coccidioses (Yamssi et al., 2017, 2018) . Therefore in fighting coccidioses, a drug with both anticoccidial effect and antioxidant activity can be more efficient, which is not the case with currently used anticoccidial drugs.
It is in this context that the Research Unit of Biology and Applied Ecology of the Department of Animal Biology at the University of Dschang has set up a research programme for several years, in which one of the aims is to promote medicinal plants used in the treatment of coccidioses. Among the plants already identified, several have been the subject of anticoccidial studies. These include the leaf of Kalanchoe crenata (Agbédé et al., 1993), Carica papaya (Mpoame Mbida et al., 2003) and Psiduim guajava (Yamssi et al., 2017, 2018).
In Dschang (West Region of Cameroon), the stem bark of P. macrophylla is used by farmers to treat bacterial infections as gonorrhoea, syphilis and typhoid, protozoan diseases such as coccidioses and malaria. Antimicrobial property and the oil extracted from the seeds are used in the preparation of formulations against pruritus, intestinal worms and dysentery (Kamanzi et al., 2002). It is on the basis of the traditional use of the stem bark of Pentaclethra macrophylla as an anticoccidial agent that we found it necessary to scientifically validate the use of P. macrophylla in fighting against Coccidiosis.
MATERIAL AND METHODS
Plant Material
The stem bark of Pentaclethra macrophylla was collected (March 2014) in Melong Littoral Region of Cameroon and identified by Mr. NGANSOP Eric, a Botanist at the Cameroon National Herbarium (Yaoundé) using a voucher specimen registered under the Reference No 2328/SRFCam.
METHODS
Preparation of Plant Extracts
Methanol, hexane and Ethyl Acetate extracts were obtained using the procedure described by Wabo Poné et al. (2012). The stem bark of P. macrophylla was air-dried at room temperature under shade in the Research Unit of Biology and Applied Ecology, and pulverized using an electrical grinder under strict hygienic conditions. One hundred grams of plant powder were macerated in 1.5 L of each of the organic solvents. This helped to extract the principal natural compounds of the plants (Wabo Poné et al., 2012). The mixture was stirred daily and 72 hours later, the resulting solutions were then filtered using Whatman Paper Number 3. The filtrate was concentrated by evaporating the solvent using a rotatory evaporator (Buchi R-200) to obtain the extracts.
For the infusion extract, a similar procedure was carried out except for the fact that distilled water was heated at 100°C and 100 g of the stored powder were poured into 1.5 L of hot distilled water. The mixture was stirred and allowed to cool for 4 hours. The resulting solutions were then filtered using a sieve and Whatman Paper N° 3. The plant extract was then distributed in large plates and the extract was concentrated by evaporating the solvent at 50°C in an oven for three days.
Anticoccidial Activities of the Extracts
Preparation of Eimeria sporulated oocysts: Field Isolates of Eimeria flavescens oocysts were collected from the large intestine while occysts of E. stiedae were collected from the gall bladders and necrotic hepatic lesions of naturally infected rabbits. Eimeria intestinalis and Eimeria magna were kindly provided by Alisson Niepceron (INRA, BASE, Tours, France). These oocysts were washed and concentrated by the flotation method. The sporulated oocysts were stored in 2.5% potassium dichromate at 4°C until they were used for experimental infections. The Eimeria flavescens, Eimeria intestinalis, Eimeria magna and E. stiedae field isolates were maintained by periodic passage through young rabbits in the Research Unit of Biology and Applied Ecology.
In vitro oocysticidal effect of extracts: One milliliter of the test solution(extract solution) was added to 1 ml of the parasitic suspension (containing 1000 unsporulated oocysts ) to give a total volume of 2 ml of each concentration of the extracts (2.5, 5, 10, 20 and 30 mg/ml) and incubated at 28°C. For comparison, phenol at 5% was used as the reference drug. The set up was examined after 24 hours and 48 hours under a light microscope using objective 40x. Twenty three Petri dishes of 5 ml were used to evaluate in vitro activities and the set up was repeated three times for each treatment and control in the same conditions. The number of sporulated oocysts (presence of four sporocysts with two sporozoites each) in the cytoplasm and non-sporulated oocysts (presence of one sporoblast in the cytoplasm) were counted and the percentage of sporulation was estimated by counting the number of sporulated oocysts in a total of 100 oocysts. The sporulation inhibitory percentage was calculated as follows.
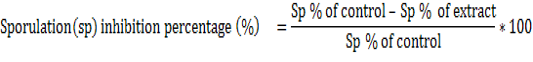
In vitro anti-sporozoidal effect of extracts: Stored oocysts in K2Cr2O7 were washed several times with Hanks buffered salt solution (HBSS) (pH 7.2) until the K2Cr2O7 was completely removed. The oocysts were then incubated in a water bath at 41oC and shaken during the incubation for 60min. The suspension was centrifuged at 3000 rpm for 10 minutes and resuspended in HBSS. Liberated sporozoites were washed with HBSS. The sporozoites were counted using the Malassez counting chamber.
Twenty three Petri dishes were used to evaluate the in vitro sporozoidal activities. One milliliter of the test solution was added to 1 ml of the parasite suspension (containing 1000 sporozoites) to give a total volume of 2 ml of each concentration of the extracts (125, 250, 500, 750 and 1000 μg/ml). For comparison, amprocox was used as the reference drug. The set up was examined after 12 hours and 24 hours and repeated three times for each treatment and control in the same conditions. The number of viable and non-viable sporozoites were counted and the percentage of viability was estimated by counting the number of viable sporozoites in a total of 100 sporozoites. The viability inhibitory percentage was calculated as follows.
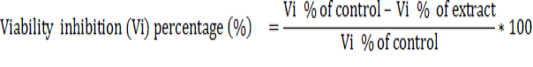
Antioxidant Activities
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay: The radical scavenging activities of crude extracts were evaluated spectrophotometrically using the 1,1-diphenyl-2- picrylhydrazyl (DPPH) free radical (El-Ghorab et al., 2006). When DPPH reacts with an antioxidant compound which can donate hydrogen, it is reduced. The changes in color were measured at 517 nm under UV/Visible light spectrophotometer (Jenway, Model 1605). Pure methanol was used to calibrate the counter. The extract (2000 μg/mL) was twofold serially diluted with methanol. One hundred microliters of the diluted extract were mixed with 900 μL of 0.3 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) methanol solution, to give a final extract concentration range of 12.5 - 200 μg/mL (12.5, 25, 50, 100 and 200 μg/mL). After 30 min of incubation in the dark at room temperature, the optical densities were measured at 517 nm. Ascorbic acid (Vitamin C) was used as control. Each assay was done in triplicate and the results, recorded as the mean ± standard deviation (SD) of the three findings were presented in tabular form. The radical scavenging activity (RSA, in %) was calculated as follows:
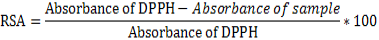
The radical scavenging percentages were plotted against the logarithmic values of concentration of test samples and a linear regression curve was established in order to calculate the RSA50 or IC50 which is the concentration of the sample necessary to decrease by 50% the total free DPPH radical (Yassa et al., 2008).
Ferric reducing/antioxidant power (FRAP) assay: The ferric reducing power was determined by the Fe3+ to Fe2+ transformation in the presence of the extracts. The Fe2+ was monitored by measuring the formation of Perl’s Prussian blue at 700 nm. Different volumes (400, 200, 100, 50, 25 μl) of methanolic extracts prepared at 2090 μg/ml were mixed with 500 μL of phosphate buffer (pH 6.6) and 500 μL of 1% potassium ferricyanide and incubated at 50°C for 20 min. Then 500 μl of 10% trichloroacetic acid was added to the mixture and centrifuged at 3000 rpm for 10 min. The supernatant (500 μl) was diluted with 500 μL of water and mixed with 100 μl of freshly prepared 0.1% ferric chloride. The absorbance was measured at 700 nm. All the tests were performed in triplicate and the results were the average of three observations. Vitamin C was used as a positive control. Increased absorbance of the reaction mixture indicated a higher reduction capacity of the sample (Noghogne et al., 2015).
Nitric oxide radical scavenging (NO) assay: The method reported by Chanda and Dave (2009) was used with slight modification. To 0.75 mL of 10 mM sodium nitroprusside in phosphate buffer was added 0.5 mL of extract or reference compounds (Vitamin C and Butylated hydroxytoluene (BHT) in different concentrations (62.5 - 1000 μg/ml). The resulting solutions were then incubated at 25°C for 60 min. A similar procedure was repeated with methanol as blank which served as negative control. To 1.25 ml of the incubated sample, 1.25 ml of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-1-napthylethylenediamine dihydrochloride in water) were added. A final concentration range of 12.5 - 200 μg/ ml (12.5, 25, 50, 100 and 200 μg/ml) was obtained. After 5 min of incubation in the dark at room temperature, absorbance of the chromophore formed was measured at 540 nm. Percent inhibition of the nitrite oxide generated was measured by comparing the absorbance values of control and test samples. The percentage of inhibition was calculated according to the following equation:
% inhibition= (1- (A1/A0) x100
Where, Al = absorbance of the extract or standard and A0= absorbance of the negative control.
Total phenol contents (TPC): The amount of total phenols was determined by Folin-Ciocateu Reagent method. The reaction mixture consisted of 20 μL of extract (2000 μg/mL), 1380 μL of distilled water, 200 μl of 2N FCR (Folin Ciocalteu Reagent) and 400 μL of a 20% sodium carbonate solution. The mixture was incubated at 40°C for 20 min. After cooling, the absorbance was measured at 760 nm. In the control tube, the extract volume was replaced by distilled water. A standard curve was plotted using Gallic acid (0-0.2 μg/mL). The tests were performed in triplicate and the results expressed as milligrams of Gallic Acid equivalents (mgGAE) per gram of extract.
Total flavonoid content (TFC): The amount of total flavonoids was determined by the Aluminum chloride method. Methanolic solution of extracts (100 μL, 2000 μg/ml) was mixed with 1.49 mL of distilled water and 30 μL of a 5% NaNO2 solution. After 5 min, 30 μL of 10% AlCl3H2O solution were added. After 6 min, 200 μl of 0.1 M sodium hydroxide and 240 μl of distilled water were added. The solution was well mixed and the increase in absorbance was measured at 510 nm using a UV-Visible spectrophotometer. Total flavonoid content was calculated using the standard catechin calibration curve. The results were expressed as milligrams of Catechin Equivalents (mgCE) per gram of extract.
Evaluation of Methanolic Extract Cytotoxicity
The cytotoxicity of the most active extract was evaluated on animal cell lines fibroblast L929 cells using MTT assay as described by Mosmann, (1983). Briefly, cells (104 cells/200 µl/well) were seeded into 96-well flat bottom tissue culture plates in complete medium (10% foetal bovine serum, 0.21% Sodium Bicarbonate (Sigma, USA) and 50 mg/ml gentamicin). After 24 hr, plant extracts at different concentrations were added and plates incubated for 48 h in a humidified atmosphere at 37ºC and 5% CO2. DMSO (10%) was used as a positive inhibitor. Thereafter, 20 μl of a stock solution of MTT (5 mg/mL in 1X phosphate buffered saline) were added to each well, gently mixed and each plate incubated for another 4 h. After spinning the plates at 1500 rpm for 5 min, supernatants were removed and 100 ml of 10% DMSO were added in each well to stop the reaction of extracts. Formation of formazon obtained after transformation of tetrazolium was read on a microtiter plate reader at 570 nm. Cell viability was determined by comparing the number of viable cells after treatment to the number of non-treated cells. The absorbance was measured at 560 nm and percentages of cell viability and the 50% cytotoxic concentration (CC50) of plant extract was determined by an analysis of dose–response curves.
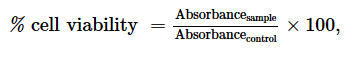
Where Absorbancecontrol is the absorbance of cells treated with DMSO 25% and Absorbancesample is the absorbance of cells treated with test sample.
Phytochemical Screening
The most active extract was tested for the presence of phenolic compounds, alkaloids, flavonoids, Polyphenols, tannins, saponin, triterpenes and steroids using standard
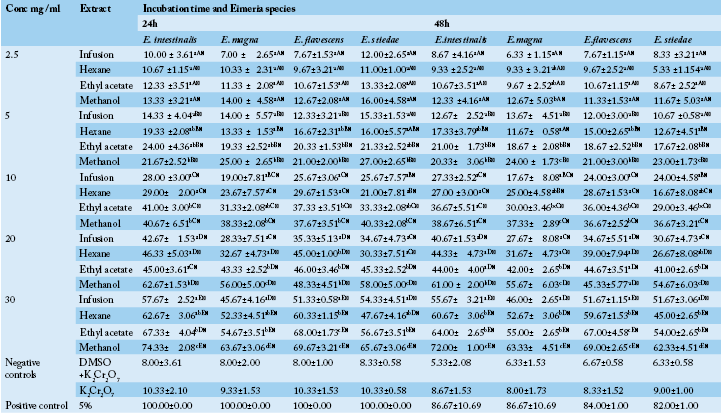
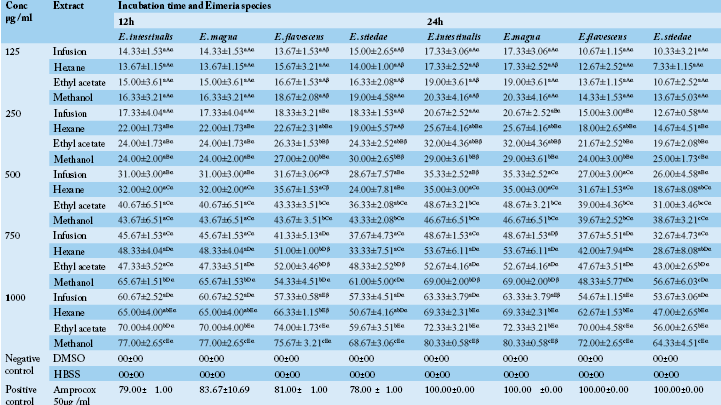
Table 1 Anticoccidial activity (Sporulation inhibition percentage) of P. macrophylla extracts on different Eimeria species as a function of concentration, incubation time and plant extract types.
ME: Methanolic extract, HE: Hexane extract, EAE: Ethyl acetate extract, IF: Infusion extract, DMSO: Diméthylsulfoxide and K2Cr2O7: Potassium dichromate. The results are the mean ± SD of triplicate tests evaluated after 24 and 48h of incubation at room temperature. a,b,c…: For the same column same concentrations, values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). A,B,C…: For the same column, same extracts and different concentrations values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). α,β: For the different times (24h, 48h), same Eimeria sp, same concentrations and same extracts, values carrying the same superscript symbol are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test).
DMSO: Diméthylsulfoxide and K2Cr2O7: Potassium dichromate. The results are the mean ± SD of triplicate tests evaluated after 24 and 48h of incubation at room temperature. a,b,c…: For the same Eimeria sp, same concentrations, values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). A,B,C…: For the same Eimeria sp, same extracts and different concentrations values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). α,β: For the different times (12h and 24h), same Eimeria sp, same concentrations and same extracts, values carrying the same superscript symbol are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test).
Table 3 : DPPH radical-scavenging activities as a function of extracts and concentration
|
Extracts
|
Concentration of extract (µg/mL) and scavenging activity (%) | IC50 (µg/ml) | ||||
| 12.5 | 25 | 50 | 100 | 200 | ||
| Infusion |
33.185±1.99aA |
37.185±0.71aB |
41.481±0.33aC |
46.666±2.18aD |
61.629±1.44aE |
787.046±115.1d |
| Hexane |
33.703±4.07aA |
38.148±1.09aB |
47.777±1.11bC |
54.518±3.90bD |
77.407±4.60cE |
182.847±82.18c |
| Ethyl acetate |
35.777±2.50abA |
61.629±1.44bcB |
79.629±1.57dC |
81.407±0.67dC |
83.037±0.55dD |
9.180±0.72a |
| Methanol |
38.296±0.84abcA |
73.163±1.96cdB |
77.407±4.60dB |
83.185±1.05dC |
85.703±0.78dCD |
6.325±1.56a |
| Vitamin C |
76.178±6,69eA |
86.186±0.62eB |
87.262±0.75eB |
90.157±1.03eC |
93.465±0.37eD |
1.295±0.14a |
a,b,c…:For the same column, values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). A,B,C…: For the same row and different concentrations values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test).
procedures described by Builders et al. (2011).
Statistical Analysis
The data obtained were analyzed using one-way analysis of variance (ANOVA) and presented as mean ± standard deviation (SD) of three replications. The levels of significance, considered at P<0.05, were determined by Waller-Duncan test using the Statistical Package for Social Sciences (SPSS) software version 12.0.
Results
Anticoccidial Activities
In vitro oocysticidal activities of extracts: The in vitro oocysticidal activity of the different extracts against Eimeria intestinalis, Eimeria magna, Eimeria flavescens and Eimeria stiedae species is summarized in Table 1. From this Table, the highest efficacy was 72.00±1.00 % at 30 mg/ml of methanolic extract against Eimeria intestinalis after 48 hours of incubation. The lowest efficacy was 7.00±4.36% (E. flavescens) at 2.5 mg/ml of the infusion extract after 48 hours of incubation. The highest efficacy of Phenol was 100% after 24 hours. Passing through the other used concentrations of P. macrophylla extracts (2.5, 5, 10 and 20 mg/ ml), they showed reduced efficacy depending on the species of Eimeria tested when compared to phenol. For each concentration and for all the Eimeria species, the methanol extract was more efficient. The general tendency was that of a decrease in inhibition rate with an increase in incubation time. The highest efficacy of tested plant extracts were recorded after 24 hours post exposure which varied according to different concentrations of the tested extracts.
In vitro anti-sporozoidal activities of extracts: Table 2 shows the sporozoidal viability inhibitory percentage of P. macrophylla extracts on different Eimeria species as a function of concentration, incubation time and plant extract types. It follows from the analysis of this Table that for each extract, an increase in concentration seemed to have enhanced its efficacy. Thus, inhibition rates significantly increased when concentration was increased. The extracts therefore have the potential of performing better at 1000µg/ml and probably at higher concentrations. According to our results, most extracts including infusion extract exhibited antisporozoidal activities against E. flavescens, E. stiedae, E. intestinalis and E. magna species at 1000µg/ml. In all the Eimeria species, methanol extract showed the highest inhibitory effect at all concentrations compared to ethyl acetate, hexane and aqueous extract (the less active). It equally appears from Table 2 that, there was an increase in inhibition rate with an increase in incubation time. A high concentration of P. macrophylla methanolic extract restricted viability percentage by 80.33±0.58 % for E. intestinalis. As the concentration of extracts decreased, the viability inhibition percentage also decreased accordingly.
In vitro Antioxidant activities of extracts
Effects of extracts on the DPPH radical: The concentrations which inhibited 50% of DPPH (IC50) are presented in Table 3. These results show that the infusion and hexane extracts of P. macrophylla all had high IC50 (low activity). The methanolic extracts had the lowest IC50 (i.e. the highest activity).
Ferric reducing/antioxidant power (FRAP) of extracts: The reducing power was determined by the Fe3+ to Fe2+ transformation in the presence of the extracts of P. macrophylla and the results obtained are shown in Table 4. From this Table, it appears that infusion extract showed the lowest reducing power while the standard (Vitamin C) exhibited the highest reducing power at the concentrations of 100 and 200 µg/ml. At 100 µg/mL, there was no significant difference between the reducing power of Vitamin C (2.510±0.65) and the methanolic extract (2.117±0.01). However, infusion extract showed the lowest optical densities (i.e. lowest reducing power) at every concentration. The remaining extracts exhibited varied activities from one extract to another at each concentration. The reducing power activity of extracts increased consistently with the increase in the concentration of extract from 12.5 μg/ml to 200 μg/ml.
Table 4 : Ferric reducing power activities as a function of extracts and concentration
| Extracts | Concentrations (µg/ml) et absorbance (à 700 nm) | ||||
| 12.5 | 25 | 50 | 100 | 200 | |
| Infusion |
0.232±0.01bA |
0.242±0.01bB |
0.402±0.03bC |
0.599±0.04aD |
0.885±0.04aE |
| Hexane |
0.383±0.01cA |
0.382±0.05cA |
0.540±0.04cB |
0.917±0.01abC |
1.291±0.07bD |
| Ethyl acetate |
0.225±0.02bA |
0.931±0.01eB |
0.954±0.06eC |
1.410±0.01bD |
1.917±0.01dE |
| Methanol |
1.291±0.07fA |
1.540±0.04gB |
1.917±0.01hC |
2.117±0.01cdD |
2.508±0.17fE |
| Vitamin C |
0.028±0.00aA |
0.044±0.00aB |
0.056±0.02aC |
2.510±0.65dD |
6.339±0.09hE |
. a,b,c…:For the same column, values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). A,B,C…: For the same row and different concentrations values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test).
Table 5 : Nitric oxide (NO) radical scavenging activities as a function of extracts and concentration
| Extracts | Concentrations (µg/ml) et pourcentage d’inhibition (%) | ||||
| 12.5 | 25 | 50 | 100 | 200 | |
|
Infusion |
79.362±0.176abA |
79.395±0.123aA |
79.526±0.102aA |
79.799±0.269abA |
79.995±0.056aA |
|
Hexane |
70.637±0.176Da |
73.611±0.546aB |
76.470±2.104aC |
77.598±1.691aC |
78.823±0.871aC |
|
Ethyl acetate |
73.169±3.141Ba |
78.202±0.857aB |
79.428±0.246aB |
79.428±0.113aB |
79.003±0.798aB |
|
Methanol |
75.457±1.643eA |
76.356±0.483aA |
78.071±11.151aB |
79.477±0.326abB |
79.542±0.157aB |
|
Vitamin C |
92.427±3.627cA |
94.595±2.032cB |
94.595±1.339bB |
96.556±0.895cC |
96.556±0.298cC |
|
BHT |
94.946±0.800cA |
96.429±0.110dB |
97.274±0.526cC |
97.624±0.027dC |
99.410±0.055dD |
a,b,c…:For the same column, values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test). A,B,C…: For the same row and different concentrations values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test).
Effects of extracts on Nitric oxide: The results of the scavenging capacity against nitric oxide were recorded in terms of % inhibition as presented in Table 5. It follows from the analysis from this Table that P. macrophylla extracts showed considerable antioxidant potential. The methanolic and infusion extracts revealed the highest percentage inhibition indicating the best nitric oxide scavenging activity. However, the hexane extract showed the lowest scavenging activity at every concentration. The extracts showed a concentration dependent NO scavenging that reached a peak of 79.99 % at 200 μg/ml of the infusion extract.
Total phenolic and flavonoid content of extracts: The total phenolic content of P. macrophylla extracts was determined in this study using Folin-Ciocateu Reagent method and the results are presented in Table 6. From this Table, it appears that the concentration of phenolic compounds in the methanolic extract (13.841 mgGAE/mg) was higher than in all other extracts. The lowest concentration of phenolic compounds was observed in the infusion extract of (3.685 mgGAE/mg). As illustrated by Table 6 below, the hexane extract had the highest flavonoid content (1.267 mgCE/mg) while infusion and the ethylacetate extracts showed the lowest value of flavonoid content (0.116 mgCE/mg).
Table 6 : Total phenolic and flavonoid contents of extracts
| Extracts | Phenols (mgGAE/mg) | Flavonoids (mgCE/mg) |
| Infusion |
3.685±0.80a |
0.116±0.01a |
| Hexane |
5.766±1.20ab |
1.267±0.13c |
| EthyllAcetate |
10.633±2.13cd |
0.116±0.01a |
| Methanol |
13.841±2.17de |
0.576±0.04b |
a,b,c…:For the same column, values carrying the same superscript letter are not significantly different at p ≥ 0.05 (Student-Newman-Keuls Test).
Cytotoxicity Activity
Table 7 shows the methanolic extract cytotoxicity concentration 50 on L929 and its selectivity index. Pentaclethra macrophylla exhibited CC50 of > 30 µg/ml, suggesting that the extract is not toxic. The selectivity index of this extract was evaluated using the MTT assay on L929 cells in order to check if its toxicity was specific to the parasite. The impact of toxicity was established by analysing the selectivity index (SI) values.
Table 7: Methanolic extract Cytotoxicity Concentration 50 on L929 and selectivity index
| CC50 (µg/ml) | Sporozoidal IC50 (µg/ml) | Selectivity index |
| 438.97 | 377.11 |
1.16 |
Phytochemical Analysis
It follows from the analysis of Table 8 that the most active extract was consistent with the detection of alkaloids, flavonoids and Tannins in the extract.
Table 8: Phytochemical screening of methanolic extract
| Chemical groups/Plant extract | P. macrophylla |
| Alkaloids | + |
| Flavonoids | + |
| Polyphenols | + |
| Tannins | + |
| Saponins | - |
| Steroids | - |
| Terpenoids | - |
+=Present, - = Absent
Discussion
According to our results, most extracts including aqueous extracts exhibited oocysticidal activity against Eimeria species. The P. macrophylla methanolic extract showed maximum sporulation inhibition activities at 30 mg/ml and were observed to be more effective against Eimeria intestinalis. Similar to present findings, Yamssi et al. (2018) observed in-vitro sporulation inhibition with Psidium guajava leaves extracts in four Eimeria species. Since condensed grape tannins have been shown to inhibit endogenous enzyme (such as mannitol-1 phosphate dehydrogenase, mannitol-1 phosphatase, mannitol dehydrogenase and hexokinase) activities (Oh and Hoff, 1986, Horigome et al., 1988), it is possible that P. macrophylla extracts (which contained tannins) reduced the rate of sporulation by inhibiting or inactivating the enzymes responsible for the sporulation process as in helminth eggs (Molan et al., 2003). Jones et al. (1994) suggested that extracts may penetrate the cell wall of oocysts and cause a loss of intracellular components. In the present study, the P. macrophylla extract might have penetrated the wall of the oocysts and damaged the cytoplasm (sporont) as evidenced by the appearance of abnormal sporocysts in oocysts exposed to higher concentrations. The observation that Potassium dichromate could not inhibit sporulation could be explained by the fact that since it is a bactericidal drug as well, it might have killed the bacteria present, thereby enhancing the sporulation of oocysts. Potassium dichromate killed bacteria in a sample containing coccidian oocysts thereby enhancing sporulation of coccidia oocysts (Hamad et al., 2014). Therefore, it could be that bacteria if present, could have interfered with the sporulation of oocysts, possibly by competing for nutrients and/or feeding on the oocysts.
The percentage of sporozoites viability under control circumstances (DMSO and HBSS) in this study was comparable with other studies using rabbits Eimeria species (Hamad et al., 2014). Our findings confirm the results of another study on the inhibitory effect of Curcuma longa on the activity of E. tenella sporozoites (Khalafalla et al., 2011). Schubert et al. (2005) have demonstrated that extracellular calcium and Ca2+ signaling are essential for the invasion of E. tenella sporozoites into host cells. Extracts have been shown to activate and desensitize receptors in calcium channels (Sarkozi et al., 2007). It is possible that P. macrophylla extracts contribute to the observed inhibition of sporozoite viability which can be by disrupting calcium-mediated signaling in the sporozoites.
According to Yamssi et al. (2018), antioxidant compounds are known to reduce the severity of Eimeria intestinalis infections in rabbits by ameliorating the degree of intestinal lipid peroxidation. The extracts significantly inhibited the activity of DPPH radicals in a dose-dependent manner and the maximum scavenging activities were observed at the concentration of 200 mg per ml. The effect of antioxidants on DPPH radical is thought to be due to their hydrogen donating ability. Hence, DPPH is usually used as a substrate to evaluate antioxidative or free radical scavenging activity of antioxidant agents. In our experiment, the high DPPH radical scavenging activities of some extracts were comparable to the standard antioxidant, vitamin C, suggestng that the extracts have some compounds with high proton donating ability and could therefore serve as free radical inhibitors. However, the organic extract of P. macrophylla except the hexane extract of P. macrophylla demonstrated a more remarkable anti-radical activity with IC50 < 20 µg/mL. In fact, according to Souri et al. (2008), the antioxidant activities of plant extracts are significant when IC50 < 20 µg/mL, moderate when 20 µg/mL ≤ IC50 ≤75 µg/mL and weak when IC50 > 75 µg/mL. There was no significant difference (p > 0.05) between IC50 values of the organic extracts and ascorbic acid except the hexane extract of P. macrophylla.
The FRAP assay, provides a reliable method to study the antioxidant activity of various extracts. In this study, the highest activity was obtained with the methanolic extract and the lowest activity was obtained with the infusion extract. These data suggest that the extract of P. macrophylla may contain several compounds with intermediate polarity. The observed reducing ability of P. macrophylla extracts in the present study could be attributed to the presence of condensed tannins as reported by Omoruyi et al. (2012). Previous studies by Omoruyi et al. (2012) and Yamssi et al. (2018) correlated the reducing power ability of plant extracts with the presence of phenols.
It is well documented that in rabbit coccidiosis, the generation of free radicals including Nitrous Oxide (NO), contribute principally to inflammatory injury, diarrhea, mortality and weight loss (Yamssi et al., 2017). There was a significant decrease in the NO radical due to the scavenging ability of extracts and ascorbic acid. The increased nitric oxide radical scavenging activity was observed in every extract of the tested plants. The infusion extract showed better scavenging capacity compared to methanolic extract. The nitric oxide scavenging potential may be due to antioxidant principle in the extract which competes with oxygen to react with nitric oxide and thus inhibit generation of nitrites (Yamssi et al., 2017, 2018).
Phenolic compounds exhibit antioxidant activity by inactivating free radicals or preventing decomposition of hydroperoxide into free radicals (Ramde et al., 2012). Flavonoid’s protective effects in biological systems are linked to their ability to transfer electrons to free radicals, chelate metals, activate antioxidant enzymes and reduce radicals
of alpha-tocopherol or to inhibit oxidases (Ramde et al., 2012). The methanolic extract of P. macrophylla was found to have high phenolic contents with 18.536 mgGAE/mg and which may be one of the reasons explaining its high antioxidant activity with an IC50 of 6.32 (DPPH radical-scavenging activity) and a Ferric reducing power activity of 2.50 at 200 µg/ml. These results suggest that phenolic compounds contribute significantly to antioxidant capacity of the investigated plant species. In addition, these results are consistent with the findings of researchers including (Raja et al., 2010, Yamssi et al., 2018) who reported such positive correlation between total phenolic content and antioxidant activity. However, Bajpai et al. (2005) disproved the correlation between phenolic compounds and antioxidant activity. Results of antioxidant assay further suggest that these extracts contain powerful free radical scavenging phytochemicals (such as phenols and flavonoids) that could be used to fight against free radical upsurge, as well as oxidative stress; and consequently might ameliorate oxidative stress-associated metabolic disorders.
Assessment of the cytotoxicity of P. macrophylla revealed that the CC50 of the methanolic extracts on L929 cell lines were above 30 µg/mL indicating the overall safety of P. macrophylla. According to Malebo et al. (2009) cytotoxicity concentrations of CC50 < 1.0 μg/ml (high cytotoxicity), CC50 1.0–10.0 μg/ml (moderate), CC50 10.0–30.0 μg/ml (mild) and CC50 > 30 μg/ml (nontoxic). We realize that, the tested extract was found to be non-cytotoxic on L929 mammalian cell lines. In a separate study, Yamssi et al. (2018) reported that methanolic extract of P. guajava lack cytotoxicity in assays involving animal cell lines fibroblast L929 using MTT assay.\
Selectivity Index is the ratio of cytotoxicity to biological activity. In our study the selectivity index values for the tested extract was 1.16. When a plant extract has a Selectivity Index value greater than one, it is more active against the target parasite strain and less toxic to the mammalian cells used in the cytotoxicity assay. On the other hand, when Selectivity Index value is less than one, it is more toxic (to the host) and less active (against the parasites). Similar results were obtained by Yamssi et al. (2018) using methanolic extract of Psidium guajava. Phytochemical screening of the most active extract showed the presence of alkaloids, flavonoids, polyphenols and tannins. This shows that flavonoids are responsible for the strong anticoccidial and antiradical activity of P. macrophylla.
Conclusion
Our findings, therefore corroborate the use of P. macrophylla as an anticoccidial agent in Cameroonian folk medicine. However, further studies to determine the in vivo anticoccidial and antioxidant activities of P. macrophylla on rabbits are needed to establish an anticoccidial prototype.
Conflict of Interest
We declare that we have no conflict of interest.
Acknowledgments
The authors wish to thank Alisson Niepceron (INRA, BASE, Tours, France) who kindly provided Eimeria intestinalis and Eimeria magna species used in the study and Dr. Michal Pakandl for his expertise in rabbit coccidioses.
Authors Contribution
This work was carried out in collaboration between all authors. Authors YC, VKP, NACN and JRK designed the study, performed the statistical analysis, wrote the protocol and wrote the first draft of the manuscript. Authors NK, EK and ML managed the analyses of the study. Authors EK and ML managed the literature searches. All authors read and approved the final manuscript.
References
tannins prepared from leaves of fodder plants on digestive
enzymes in vitro and in the intestine of rats. Brit. J. Nutr. 60:
275–285 https://doi.org/10.1079/BJN19880099
in vitro activity of digestive proteases and activation of their zymogens. J. Food Sci. 51: 577–580. https://doi.org/10.1111/j.1365-2621.1986.tb13883.x