The Journal of Advances in Parasitology
Research Article
Impact of Nematodes on the Gastric Mucosa of the American Alligator (Alligator mississippiensis)
Rekha R. Yesudas1*, Bruce A. Young2
1Department of Pharmacology, Kirksville College of Osteopathic Medicine, A.T. Still University, Kirksville, MO, 63501, USA; 2Department of Anatomy, Kirksville College of Osteopathic Medicine, A.T. Still University, Kirksville, MO, 63501, USA.
Abstract | The intestinal tract represents an ideal habitat for a large number of parasites, as compared to stomach’s acidic environment. The American alligator (Alligator mississippiensis, Daudin) has “typical” vertebrate gastric pits and glands in the stomach mucosa, with a corresponding acidic gastric environment. Crocodylians have a remarkably effective immune system, which enables them to combat microbial infections and allows relatively quick wound healing. We sought to explore how the alligator’s immune system responded to parasites, rather than microbial, challenges. We examined the stomachs of 14 sub-adults, wild-caught, American alligators from the coastal region of Louisiana, and found that four (~29%) were infected with enteric helminthes. Two different nematodes were found: Dujardinascaris waltoni (Nematoda: Heterocheilidae) was found loose among the stomach contents, whereas Ortleppascaris antipini (Nematoda: Ascaridoidea) was associated with multifocal lesions in the gastric mucosa. These lesions were roughly 4 mm in diameter, housed multiple parasites, and formed an elevated node of mucosa and nematodes. As the parasites invaded the gastric mucosa, they induced a granulomatous inflammation near the lesion; the site of worm attachment was associated with a marked eosinophilic necrosis. This cellular response produced a penumbra around the invasive nodule that was clearly visible under light and electron microscopy. Embedded worms were surrounded by an eosinophilic exudation, presumably representing proliferating host mucosal tissue, which formed the elevated nodule. Mucosal burrows of the nematodes led to a loss of the gastric pits in the infected zone and a presumed decrease in digestive efficiency of the host alligator.
Keywords | Stomach, Crocodylia, Parasite, Lesions, Inflammation, Necrosis
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 21, 2020; Revised | June 02, 2020; Accepted | June 05, 2020; Published | June 26, 2020
*Correspondence | Rekha R. Yesudas, Department of Pharmacology, Kirksville College of Osteopathic Medicine, A.T. Still University, Kirksville, MO, 63501, USA; Email: [email protected]
Citation | Yesudas RR, Young BA (2020). Impact of nematodes on the gastric mucosa of the American alligator (Alligator mississippiensis). J. Adv. Parasitol. 7(2): 7-13.
DOI | http://dx.doi.org/10.17582/journal.jap/2020/7.2.7.13
ISSN | 2311-4096
Copyright © 2020 Panicker et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Parasites have evolved tremendous strategies that allow them to persist and spread in a variety of hosts, and host tissues. The vertebrate stomach is an unusual habitat for parasites due to the acidic environment, which can digest softest bodied organisms (Webber, 2015). Accordingly, most gastrointestinal parasites are reported from the vertebrate intestine, rather than the stomach (Garcia, 2018). Stomach flukes are trematode parasites, commonly found in the dolphin fish Coryphaena hippurus (Rekha and John, 2004), which rely on a thickened tegument to avoid digestion. Nematodes have a collagenous cuticle as the outer integument layer, which allows some species to survive in the acidic environment of the vertebrate stomach (Stepek et al., 2007). They inhabit a variety of hosts, such as fish, amphibians and turtles, which are regularly consumed by opportunistic predators like alligators (Keenan et al., 2013).
Alligators and crocodiles live in unsanitary environments with constant exposure to pathogens. Crocodylians live with opportunistic infections, but their immune system helps them avoid adverse effects even if their wounds are exposed to foul water (Kommanee et al., 2012). Crocodiles in turbid swamp waters are resistant to infections due to the antibiotic properties of their blood plasma (Kommanee et al., 2012; Criscitiello et al., 2020). The innate immune system of Crocodylians is composed of non-specific leukocytes, antimicrobial peptides and the complement system (Zimmerman et al., 2010). Key components of this system shown to have anti-microbial functions include: leukocyte extract (Merchant and Britton, 2006; Merchant et al., 2004; 2005a, b), white blood cell extract (Phosri et al., 2018), cationic peptides (Bishop et al., 2015), and beta-defensin peptides (Tang et al., 2018).
Nematodes in the genus Dujardinascaris and Ortleppascaris are reported to be found in the stomach and intestine of Crocodylians. Two species of Dujardinascaris are reported in the Alligator mississippiensis gastrointestinal tract: D. helicina and D. waltoni (Sprent, 1977; Tellez, 2013). Ortleppascaris antipini is the only species which has been reported in A. mississippiensis gastrointestinal tract (Sprent, 1978; Waddle et al., 2009). Gastrointestinal nematode infections in Crocodylian hatchlings are generally asymptomatic but may be occasionally associated with runting (Ladds and Sims, 1990). Adult Crocodylians may be infected by a number of trematodes, which resulted in poor health and growth rate (Perez-Benitez et al., 1980). Little is known about the stomach parasitism and host immune response in A. mississippiensis; the present study was undertaken to document the alligator’s immune response against O. antipini gastric invasion.
MATERIALS AND METHODS
Live animals
Fourteen live sub-adult (165–183 cm total length) wild caught American alligators, A. mississippiensis, were obtained from the Louisiana Department of Wildlife and Fisheries. The animals were housed communally in a 29 m2 facility that featured three submerging ponds, natural light, and artificial lights on a 12:12 cycle. The facility was maintained at 30 – 33oC, warm water rain showers were provided every 20 minutes which helped maintain the facility at > 75% relative humidity. The alligators were maintained on a diet of previously frozen adult rats. The husbandry and use of the live alligators followed all applicable federal guidelines, and was approved by the IACUC of A.T. Still University (Protocol #209, approved 21 March 2018).
Collection of the parasites and host tissue
At the conclusion of non-related experimental studies, the alligators were anesthetized with isoflourane and euthanized by cardiac excision. The stomach and intestinal tract was examined immediately after euthanasia. Nematodes found free in the stomach were collected, washed in PBS, then fixed in hot 70% ethanol. Portions of the stomach wall bearing visible lesions and nematodes were excised (with the parasites still in situ), rinsed in PBS, and fixed in NBF. Subsequently, both groups of nematodes were cleared in lactophenol (5ml liquid phenol, 5ml lactic acid, 10 ml glycerol, 5 ml distilled water with cotton blue) for identification.
Scanning electron microscopy (SEM)
NBF-fixed tissue samples of alligator stomach mucosa, with and without embedded nematodes, were dehydrated with an ethanol series then treated with 1% osmium tetroxide. Samples were examined using a TM3000 scanning electron microscope (Hitachi High-Technologies Corp., Northridge, CA).
Histological preparation
Stomach wall samples of A. mississippiensis with and without parasites were dehydrated through an ethanol series then embedded in paraffin. The 10µm sections were mounted then stained with hematoxylin and eosin following Luna (1968). Histological material was examined and photographed using a DM 4000B microscope (Leica Microsystems Inc., Buffalo Grove, IL).
RESULTS
Nematodes were found in three of the 14 (29%) Alligator mississippiensis. Two different parasites were found; Dujardinascaris waltoni (Nematoda: Heterocheilidae) was found loose in the stomach lumen, while Ortleppascaris sp. (Nematoda: Ascaridoidea) was found embedded in the stomach wall. The D. waltoni ranged from 2.5 to 3 cm long (Figure 1). The Ortleppascaris ranged in length from 1.0 to 1.5 cm (Figure 2). The Ortleppascaris were found embedded into the mucosa at focal nodules, each nodule was approximately 4 mm in diameter with multiple worms protruding from deep ulcers (Figure 2).

Figure 1: Live Dujardinascaris waltoni isolated from the stomach lumen of Alligator mississippiensis.
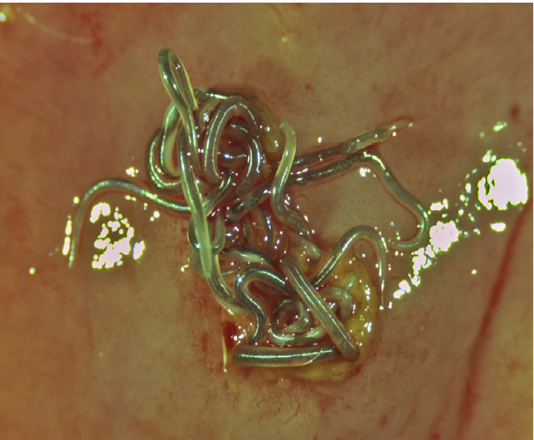
Figure 2: Superior view of a group of live Ortleppascaris antipini on a freshly excised portion of the stomach wall of Alligator mississippiensis. The nematodes are embedded in the mucosa at a focal nodule approximately 4 mm in diameter.
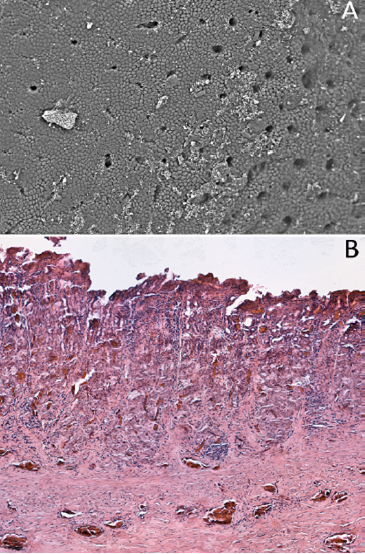
The impacted worms burrowed into the mucosa, where tissue damage was grossly visible with pit formation (Figure 3). Hemorrhagic exudate and cellular debris were found in the sunken pit with a distinct border (Figure 3). Structural damage to the mucosa and submucosa was evident with regeneration of partially repaired submucosa. The simple columnar epithelial layer of the gastric mucosa was destroyed completely in the lesioned zone extending to the connective tissue layer of the submucosa. Inflammatory exudation filled the eroded zone (Figure 3). The muscular layer and tunica adventitia remained intact.
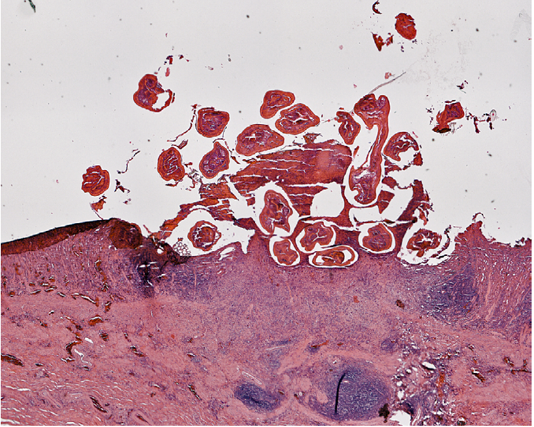
Figure 3: Section of the stomach wall of Alligator mississippiensis showing a lesion about 4 mm in diameter, housing multiple Ortleppascaris antipini.
Lesions were surrounded by a penumbra of mucosal damage (Figure 4A). Eosinophilic exudation and mononuclear infiltration, presumably representing proliferating host mucosal tissue responding to the parasitic challenge, were evident (Figure 4B). This response resulted in an elevated nodule of worms and mucosa in the gastric wall. In the present case, it was not clear whether pre-existing inflammation allowed the entry of the nematode into the stomach wall or the presence of parasite led to the inflammation and subsequent lesion.
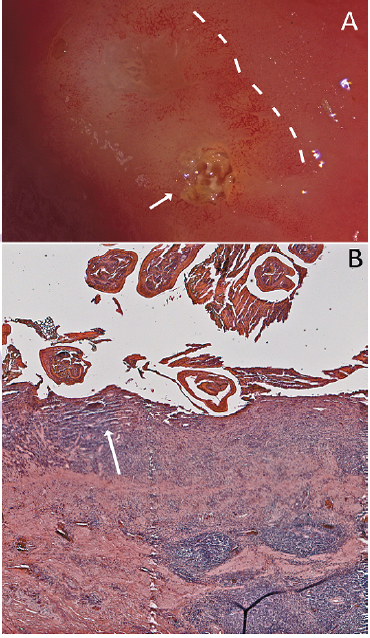
Figure 4: Mucosal response to the nematode invasion. A) Photograph of excised stomach wall of Alligator mississippiensis; the embedded Ortleppascaris have been removed from the lesion. The lesions (arrow, right) caused a penumbra of mucosal damage that was clearly visible (dashed lines); B) Section of the stomach wall of Alligator mississippiensis showing the gastric mucosa invaded with the Ortleppascaris. The parasites invaded the mucosa and submucosa, triggering a granulomatous inflammation response and a marked eosinophilic necrosis (arrow).
Alligators have “typical” vertebrate gastric pits and glands (Figure 5) in the stomach mucosa. The granulomatous inflammation and eosinophilic necrosis induced by the nematodes led to a loss of the gastric pits in the infected zone (Figure 6).
DISCUSSION
Parasites in the digestive tract are mainly in the intestinal lumen, only rarely in the acidic vertebrate stomach (Rekha and John, 2004). Alligators and crocodiles digest their food by concentrated hydrochloric acid, proteolytic enzymes, rhythmic gastric contractions and the grinding effects of gastroliths (Seah et al., 2017). Herein we report the presence of nematodes from the genera Dujardinascaris and Ortleppascaris in the alligator stomach. In some nematodes collagenous and non-collagenous cuticle proteins resist solubilization even under strong denaturating conditions, allowing the nematode to resist the stomach’s hostile environment (Fetterer and Rhoads, 1993). Dujardinascaris waltoni has been reported from the intestine and stomach of the American crocodile (Crocodylus acutus) and American alligator (A. mississippiensis) (Sprent, 1977; Tellez, 2013). 96% of enteric distribution of Dujardinascaris sp. in A. mississippiensis was reported in the stomach, and only 4% in the intestine (Hazen et al., 1978). Our findings support the incidence of D. waltoni in the lumen of the alligator’s stomach.
Nematodes have not been previously described to burrow into the Crocodylian stomach wall. This pathology was observed in multiple specimens of A. mississippiensis examined for this study. Multiple clusters of Ortleppascaris antipini were seen embedded in the A. mississippiensis stomach wall (Figure 2) creating inflammatory lesions (Figure 2). Previous studies have described keratitis and enteritis associated with nematode infections in Crocodylians, but similar reports are lacking from A. mississippiensis. Nematode infected young captive crocodiles were reported with cutaneous ulcers, stromal keratitis, and enteritis (Ladds and Sims, 1990). Zhao et al. (2015) reported superficial ulceration of gastric mucosa and submucosal inflammation in the entire gastric wall of a Chinese alligator (A. sinensis) infected with O. sinensis.
The cylindrical, funnel-shaped, gastric pits in alligators (Figure 5) are characterized by typical columnar cells (Luppa, 1977). Parasitic infections can result in a chronic hyperplastic condition characterized by granulomatous inflammation (Shah et al., 2017). In the present study isolated gastric tissue from A. mississippiensis infected with Ortleppascaris antipini showed an inflammatory reaction resulting in granulomatous lesions (Figure 4). Focal areas showed hemorrhage and sloughed out zones surrounded by zone with eosinophil rich inflammatory infiltration in the serosa (Figure 4). The alligators studied were healthy without any of the clinical signs of crocodylian nematode infection including anemia, anorexia, bloat, regurgitation, signs of obstruction and wasting disease (Lane and Mader, 1996). In other hosts, nematodes are known for extraintestinal migration, leading to a range of complications and diseases (Ghazala et al., 2015; Sohn et al., 2015), no evidence of extra intestinal migration was seen in the specimens of A. mississippiensis examined.
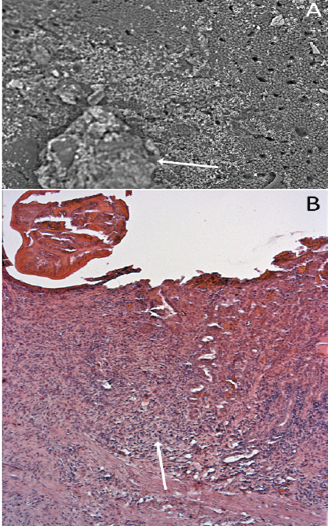
Figure 6: The influence of nematode infection on the gastric glands and pits of Alligator mississippiensis. A) Scanning electron micrograph showing the stomach wall of Alligator mississippiensis. The Ortleppascaris infection (arrow) has produced a central area of necrosis and a penumbra of mucosal damage; B) Section of the stomach wall of Alligator mississippiensis showing the loss of the gastric pits in the area of the Ortleppascaris invasion (arrow).
Alligator mississippiensis which inhabit inland freshwater were reported to have more O. antipini than those from coastal esturine habitats (Tellez and Nifong, 2014). The relative abundance of the nematodes reflects the combined influences of the ecological and phylogenetic diversity of the primary host species, and the opportunistic predation of A. mississippiensis (Tellez and Nifong, 2014). The present study revealed a novel strategy in O. antipini to withstand the hostile environment of the alligator stomach; embedding into the stomach wall. This example of parasitic adaptation is associated with a specialized host response; the alligator’s immune system showed a significant granulomatous inflammation at the site of nematode infection.
Alligator mississippiensis might be an accidental host of O. antipini. The gastric mucosa of the alligator specimens we examined revealed no existing ulcers that were not associated with O. antipini; however, we cannot exclude that these parasites were exploiting pre-existing ulcers. This study found two enteric nematodes in A. mississippiensis (Dujardinascaris waltoni and Ortleppascaris antipini). Little is known regarding the seasonal abundance of these parasites in crocodylians. A. mississippiensis is an opportunistic and intermittent forager, which often fast between October to March (Joanen and McNease, 1987). During these fasting periods the stomach acidity maintains an average pH of 2.6 (Joanen and McNease, 1987), so the enteric parasites would still be exposed to a chemically hostile environment but would have only the alligator’s tissue for nourishment.
Recently, there has been considerable interest in the host-parasite relationship/response of the order Crocodylia due to the antineoplastic and antibiotic properties of crocodile blood. Crocodile blood extract induces apoptosis in lung cancer cells (Ou and Ho, 2016). Serum and organ lysates of Crocodylus palustris have powerful antiamoebic and antitumor activity (Siddiqui et al., 2017). We hope to investigate the interaction(s) between A. mississippiensis plasma and corpuscles in the granulomatous inflammation, and the collagen and non-collagenous proteins in the cuticle of O. antipini.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Ruth Elsey and the Louisiana Department of Wildlife and Fisheries for the alligators used in this study, as well as P. Kondrashov and K. Elmslie for their continued support.
AUTHORS CONTRIBUTION
The authors contributed equally.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
REFERENCES