Advances in Animal and Veterinary Sciences
Research Article
Detection of Rabies Virus and its Pathological Changes in Brain of Buffaloes in Egypt
Sahar Abd El Rahman1*, Mohamed Elbeskawy2, Mohammed Fawzy Hamed3
1Department of Virology; 2Department of Internal Medicine, Infectious and Fish Diseases; 3Department of Pathology, Faculty of Veterinary Medicine, Mansoura University, 35516 El-Gomhoria street, Mansoura, Egypt.
Abstract | This study aims to detect the rabies viral antigen in the brains of naturally infected buffaloes by immunofluorescence and electron microscope, identify the histopathological and ultrastructure alterations of infected brain concerning the changes in endothelial cells of the blood barrier and evaluate rabies cycling in Egypt. Necrotic brains were carefully collected from ten expected to be rabid infected buffaloes, evaluated for gross lesions of rabies disease and microscopically examined for viral antigen expressions and histopathological changes. The results of the gross examination of the collected brains showed the presence of severe congestions in both meningeal and cerebral blood vessels, as well, hemorrhage and edema in cerebrum and cerebellum. The immunofluorescence and electron microscopes revealed the presence of the rabies viral antigen, Negri bodies and the characteristic bullet shape of the viral particles. Histopathological examination estimated the presence of intracytoplasmic inclusion bodies (Negri bodies) in the Purkinje cells of cerebellum, vasculitis, necrotic endothelial cells and marked neuronal necrosis with satellitosis and neuronophagia. The electron microscopic reported vacuolated endothelial cells, clumped nuclear chromatin of loss tight junctions, and vasogenic edema with consistent erythrocytes leakage in the infected brain. We concluded that rabies infection of buffaloes could be easily diagnosed in the laboratory by fluorescence and electron microscopes. Pathologically, rabies infection could cause injuries of the endothelial cells with disruption of blood brain barrier causing detrimental alterations. Special attention should be given to stray dogs and outdoor rearing of buffaloes.
Keywords | Rabies, Buffaloes, Immunofluorescence, Electron microscope, Blood brain barrier
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 06, 2015; Revised | September 21, 2015; Accepted | September 22, 2015; Published | October 12, 2015
*Correspondence | Sahar Abd El Rahman, Mansoura University, 35516 El-Gomhoria street, Mansoura, Egypt; Email: [email protected]
Citation | Abd El Rahman S, Elbeskawy M, Hamed MF (2015). Detection of rabies virus and its pathological changes in brain of buffaloes in Egypt. Adv. Anim. Vet. Sci. 3(11): 588-593.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.11.588.593
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Abd El Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Rabies virus (RABV) belongs to genus Lyssavirus, family Rhabdovirus, causes an acute, fatal, neurological disease of the central nervous system, spreads among domestic dogs and wild carnivorous animals by biting and infects all warm-blooded animals including humans. RABV is a “type species” virus, enveloped and has a single stranded, negative sense RNA genome, the Greek name Rhabdo describes the characteristic bullet or rod-shape of the rabies virus detected by the electron microscopy (Drew, 2004). The world health organization (WHO) currently reports , the fluorescent antibody test (FAT) to be as the best test for detection of rabies viral antigen using a conjugated monoclonal antibody against the rabies nucleocapsid protein (Hayman et al., 2011), which appears as fluorescence of specific aggregates (Shankar, 2009).
The causative virus is often excreted in the salivary glands of rabid animals, thus the bite of the infected animal introduces the virus into a fresh wound. The incubation period for rabies is broad, ranging from a few weeks to one year that relates to several variables, including the site of the inoculum and the viral load associated with the trauma (Bahmanyar et al., 1978; Depowski et al., 2002). RABV replicates in muscle cells prior to the invasion of the peripheral nervous system and central nervous system (Murphy and Bauer, 1974), it propagates along nerve tissue from the wound to the brain causing serious neurological manifestations in the central nervous system. RABV replicates in the large neurons of the brain as pyramidal neurons of hippocampus of dog and Purkinje cells of cow and buffalo forming clear eosinophilic intracytoplasmic pathognomonic lesions called Negri bodies (Jamadagni et al., 2007).
Once inside the CNS, rapid dissemination of the virus occurs with serious pathological changes in the brain as neuronal necrosis, neuronophagia, satellitosis, and perivascular cuffing and rapid progressive encephalitis ensues (Fishbein and Bernard, 1995). Rabies induces fatal encephalitis which is considered to be the key event in the death of human and animals, where rabies encephalitis induces the liberation of inducible nitric oxide, which has a deleterious effect on the brain tissue (Ubol et al., 2001). Additionally, Tumor necrosis factor alpha (TNFα) expression is suggested to be an important indicator in rabies encephalitis infection (Marquette et al., 1996; Camelo et al., 2000).
The blood-brain barrier (BBB) is a monolayer of cells that regulates the passage of solutes between the CNS and the blood. The endothelial cells that form the barrier at the capillaries, venules and arterioles control the homeostatic environment of the CNS (Banks, 1999), The BBB is formed by a complex cellular system of endothelial cells, astroglia, pericytes, perivascular macrophages and a basal membrane, although the anatomic substrate of the BBB is the inter-endothelial tight junctions that form a continuous sealing (Weiss et al., 2009).
Buffalo is an important species reared in the Egyptian farms and villages; they usually are exposed to biting by rabid dogs and foxes causing fatal nervous manifestations with eventual death. The previous studies pointed that the histopathological changes on brains were mild in correlation to severe neurological manifestations of the rabies infected animals (Jamadagni et al., 2007). In this study, we concerned to detect the viral antigen by fluorescence techniques, evaluate the characteristic histopathological and ultrastructure alterations of the infected brains and investigate the effect of rabies on the endothelial lining of brain blood barriers of infected buffaloes.
MATERIALS AND METHODS
Animals
Ten brain samples of expected to be rabid buffaloes from different localities in Dakahlia government were collected during the period of (March 2012-September 2014), with and without a history of dogs biting from about one to three months. All examined buffaloes suffered from ataxia, hyperesthia, severe bellowing, with foamy salivation and paralysis. They were examined clinically after death with special care, according to Kelly (1996). Necropsies were collected for five cases at the department of Pathology, Faculty of Veterinary Medicine, Mansoura University, Egypt.
Collection of Samples and Gross Examination
The brains were collected from the ten rabid expected buffaloes, carefully examined for post mortem gross lesions and some selected samples were partially divided for preparation of cryo- and paraffin sections. All samples were directly immersed in 10% buffered formalin saline and 5% glutaraldehyde for histopathological, immunofluorescence and electron microscopic investigations respectively according to Li and others (2005).
Immunoflourescent Technique
Parts of fresh infected brains were frozen, sectioned (cryo-sections) and used for FAT. All slides were immersed in anti-rabies FITC conjugated antibody (BBL Anti-Rabies Globulin, fluorescein labeled; Becton Dickinson, Cockeysville, MD) at a final dilution of 1:80 according to manufacturer’s instructions. All slides were kept in a dark room at 37oC for 45 min., washed thrice using Phosphate buffered saline (PBS) and dried at room temperature. Examination of all slides by the fluorescence microscopy was done (magnification x400) (Warner et al., 1997).
Histopathological Examination
After fixation in 10% buffered formalin for 48 hours, the tissues were thoroughly washed in running water, dehydrated in ascending grades of alcohol and acetone, cleared in benzene and embedded in paraffin at 58oC. The paraffin embedded tissues were sectioned at five microns thickness and stained by haematoxylin and eosin according to (Luna, 1968). The tissues were screened for the presence of various histopathological changes by light microscope (Olympus).
Transmission Electron Microscope
Pinhead sized brain samples were fixed in 5% glutaraldehyde buffer for 24 hours, washed three times in cacodylate buffer (0.1M, pH 7.2) each for 20 min., and fixed in 1% osmium tetroxide for 2 hours. Samples dehydration was done by immersing them in ascending grades of ethyl alcohol up to 100% (30, 50, 70, 80, 90 and 100% each for 2 hours). Using the gelatin capsule, samples were embedded in Epon 812, kept in an incubator at 35˚C for one day, 45˚C for another day and 60˚C for three days for polymerization (Bozzole and Russell, 1999). Semi-thin sections (0.5-1μ thick) were prepared, stained with toluidine blue and examined by light microscope (sc30 Olympus camera). Regions for preparation of ultrathin sections were oriented. The ultrathin sections at a thickness of 500-700 Å were prepared by using Leica AG ultramicrotome and contrasted with uranyl acetate for 15 min., followed by lead citrate for 5 min. examined by a TEM 100 CX11electron microscope at 80 kv and photographed by CCD digital camera model XR-41.
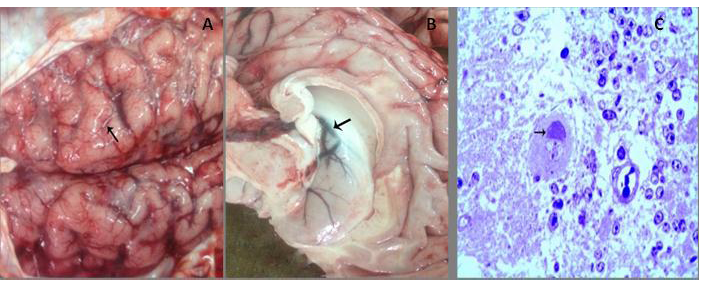
A) Congestion of meningeal blood vessels with flattening gyri and narrowing sulci (arrow); B) Congestion and edema in the wall of the lateral ventricle (arrow) with bulging into the lumen. C) Bluish stained Negri body forming a cap over the nucleus with interstitial edema. (Toluidine blue, x400)
RESULTS
Clinical Examination of Buffaloes
All examined buffaloes suffered from severe nervous manifestations, staggering, hyperesthesia, kicking the ground, continuous bellowing, profuse salivation, stasis of rumination and rapid heart rates. Some buffaloes had unsteady gait and recumbency with congested mucous membrane and shallow respiration and bradycardia.
Gross Examination of Infected Brains
The post-mortem revealed severe congestion of meninges, cerebellum and cerebral hemisphere with hemorrhage. Edema of the cerebellum and cerebral hemisphere, in addition to, widening of gyri and narrowing of sulci of cerebral hemisphere were seen (Figure 1A). Moreover, severe congestion and edema of the lateral ventricle that leading to bulging of the wall into the lumen (Figure 1B).
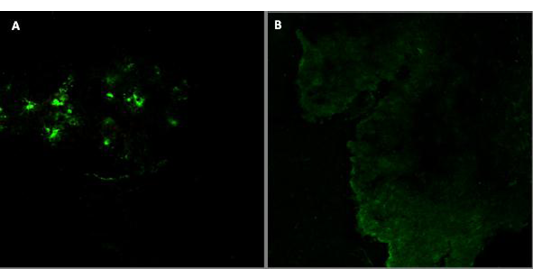
A) Positive reaction in infected brain tissues; B) Control brain tissue of non-infected tissue
Immunoflourescence Detection of Viral Antigen
Staining of the cryo-sections of infected brains by fluorescence techniques revealed the presence of rabies antigen in the brain cells (Figure 2A), while no antigen expression was detected in the control of the buffalo’s brains (Figure 2B).
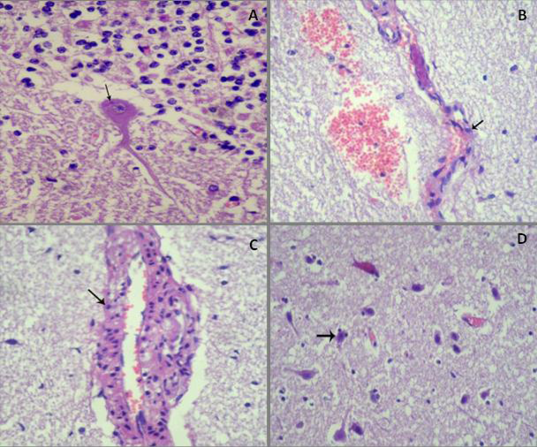
A) Esinophilic intracytoplasmic inclusion body (Negri body) around nucleus of Purkinje cell, (HE, x400); B) Endothelial cells become round (arrow) with necrosis and desquamation, perivascular edema and hemorrhage, (HE, x100); C) Swollen endothelial lining of cerebral capillaries and perivascular edema with lymphohistiocytic infiltration in its wall (arrow), (HE, x400); D) Edema of neuropil and status spogiosus, in addition to diffuse neuronal necrosis and satellitosis (arrow) ((HE,x100)
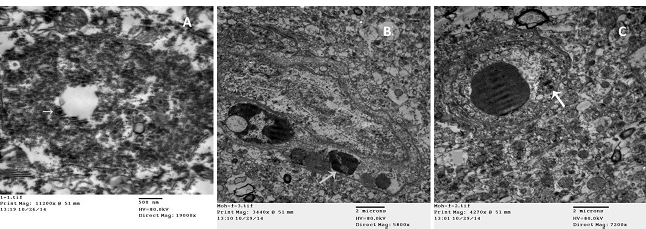
A) Electron dense intracytoplasmic inclusion with bullet shape viral particles (arrow) (TEM); B) Clumped chromatin of endothelial cell (arrow), with vasogenic edema (TEM); C) Chromatolysis and vacuolation of endothelial cell (arrow), with vasogenic edema (TEM).
Histopathological Investigations
Histopathological investigations estimated the presence of eosinophilic intracytoplasmic inclusion bodies (Negri bodies) in the cytoplasm of the purkinje cells, either in perikaryon (Figure 3A) or in cytoplasmic process. The detected Negri bodies varied in size and clearly appeared in eight samples while in the other two samples were unclear. The endothelial lining of the cerebral capillaries was swollen, necrotic (Figure 3B), vasculitis and lymphohistiocytic infiltration into the wall of cerebral blood vessels were the consistent lesions (Figure 3C), in addition to marked vasogenic edema and diffuse hemorrhage into brain tissue. Neuronal necrosis, satellitosis, gliosis, neuronophagia, edema of the neuropil and status spongiosus were observed (Figure 3D). Lymphocytic infiltration in the meninges with congestion of meningeal blood capillaries was noticed.
Toluidine Blue Stained Sections
The Toluidine blue staining showed the presence of bluish stained round to oval intracytoplasmic inclusions of varying size and form cap over the nucleus of Purkinje cells (Figure 1C). Marked edema, erythrocytes leakage into the brain parenchyma and damage of the endothelial lining of blood capillaries were investigated.
Transmission Electron Microscopy
The electron microscope, used for transmission scanning of the infected brains of buffaloes, could detect electron dense intracytoplasmic inclusion bodies, where dense viral particles with hallo area and double coat appeared as bullet shape surrounded by rough endoplasmic reticulum (Figure 4A). Severe vacuolation in the cytoplasm of endothelial cells with clumped chromatin and chromatolysis of its nucleus were investigated (Figure 4B and 4C), vasogenic edema in brain tissue and the escape of erythrocytes with damaged brain tissue.
DISCUSSION
Rabies is a highly important zoonotic disease that is widespread in different animals, causing progressive encephalitis. The admitted rabies infected buffaloes expressed different nervous signs with a history of dogs’ biting in most examined buffaloes as the main source of infection which confirm the natural route of spreading RABV infection, while those expressed RABV infections without a history of biting by dogs might be infected by the biting of other wild carnivores as foxes. The wild carnivores might attack the buffaloes mostly reared near to the margins of the villages (Mitmoonpitak et al., 1997; Cistema et al., 2005). Although the appearance of nervous manifestations, the history of biting from street dogs in most recorded cases and the clinical observations are not characteristic and may differ greatly from one animal to another, as well, the atypical nature of the disease which is not easy to be predicted by clinical signs. Rabies should be considered in any animal that suddenly develops profound behavioral changes, and/or features of limb paralysis. Post-mortem examinations of the collected brains with a reliable laboratory diagnosis of rabies are required for identifying either the whole virus or some of its specific components.
Both of WHO and OIE recommend FAT as the most widely used test for rabies diagnosis. This ‘gold-standard’ test may be used directly on a smear, and can also be used to confirm the presence of propagated rabies virus in cell culture or brain tissue of mice inoculated for diagnosis (Shankar, 2009). The FAT is a sensitive, specific and cheap laboratory diagnostic tool for detection of RABV; its sensitivity depends on the degree of autolysis of the tissues and the way of the brain sampling (Barrat and Aubert, 1995). The FAT is also used for the specimens preserved in a formalin solution after treatment of the specimens with a proteolytic enzyme (Warner et al., 1997).
The characteristic Negri body in the cytoplasm of Purkinje cells of the cerebellum is an important diagnostic lesion of rabies infections of buffaloes which indicates rabies disease (Archana, 2001; Jamadagni et al., 2007). The Negri body was small in one cell and became larger in another one, in correlation with electron microscopic features that explains replication of virus particles (Matsumoto, 1963).
Endothelial cell injury was the main pathological lesion noticed in all cases which may be attributed to rabies encephalitis. This injury was probably caused by the released cytotoxic mediators that have great influence on endothelial cells as TNF (Nuovo et al., 2005). This conducted with a recent report demonstrated that increased brain endothelial permeability in vitro via TNF-α that leads to endothelial disorganization with human immunodeficiency viral infection (Afonso et al., 2007).
Presence of vacuolation in endothelial cells contributes to disruption of the blood brain barrier (Hansson et al., 1975; Mayhan and Heistad, 1985) also, degenerative changes and necrosis of endothelial cells leads to loss of tight junction between them are a major cause for BBB dysfunction, often referred to as “BBB opening”, has long been known to constitute a key feature of the progression of several CNS diseases, as a consequence of Neuro-inflammation (Weiss et al., 2009).
Severe vasogenic and interstitial edema in the brain appeared by accumulation of extracellular fluid which increased the pressure on brain tissue with subsequent great pathological alteration as neuronal dysfunction (McGavin and Zachary, 2007), this attributed to endothelial cells damage allowing fluid leakage into brain tissue with subsequent vasogenic edema.
Comparable to other studies we conducted that encephalitis induced were mild, but has great pathological effect on the endothelial cells forming BBB dysfunction leading to progressive pathological alterations in the brain. This study declared that in addition to replication of Lyssa virus on infected cells as Purkinje cell. Endothelial is highly vulnerable to injury by rabies encephalitis with detrimental pathological alterations in the brain of infected buffaloes, reflected by fulminant fatal course of disease on rabid buffaloes. Confirmed diagnosis of rabies infection of buffaloes without a history of dog biting gives an announcement about other sources for rabies occurrence in animals. In Egypt, no eradication strategy is used for controlling the spreading of rabies infection by strict vaccination programs to street dogs, as well, no continuous evaluation for the sources of spreading rabies infection between animals. Further study is running to characterize the genotype of the collected samples of rabies virus by molecular techniques, comparing the current circulating type in Egypt with that in the neighbor countries and the reference known types.
Conflict of interests
The authors declare to have no conflict of interest.
Authors’ contributions
S. Abd El Rahman designed and carried out the experiments. M. Elbeskawy collected the field samples. M. Hamed carried out the histopathological examinations. All authors wrote and approved the final manuscript.
ACKNOWLEDGMENTS
Authors are thankful to Prof. Dr. Emad Younise, Department of Internal Medicine, Infectious and Fish Diseases, Mansoura University, Egypt for the field diagnosis of rabies and samples selection.
References