Advances in Animal and Veterinary Sciences
Research Article
Histomorphological and Biochemical Studies in Ovaries of Female Tatera indica and Bandicota bengalensis Inhabiting South-West Region of Punjab in North India
Parkash Singh1*, Gurinder Kaur Sangha2
1Department of Medical and Biotechnology, Baba Farid College, Bathinda-151001, India; 2Department of Zoology, Punjab Agricultural University, Ludhiana-141004, India.
Abstract | The Malwa region of Punjab, India is facing an unprecedented crisis of environmental health linked to indiscriminate, excessive and unsafe use of pesticides, fertilizers, and poor groundwater quality. Studies of this region have also highlighted a sharp increase in many other pesticide-related diseases such as mental retardation and reproductive disorders. The present study was designed to examine the adverse effect of environmental contaminants on the female fertility indices in Tatera indica and Bandicota bengalensis inhabiting South-West region of Punjab in North India. Significant decrease in the weight of ovary in Tatera indica and increase in the weight of vagina was observed in female rats Tatera indica and Bandicota bengalensis collected from Bathinda region as compared to the Ludhiana rats. The amount of total proteins, total lipids, phospholipids and cholesterol and the level of various ovarian enzymes like acid phosphates (ACP) and alkaline phosphates (ALP) decreased in ovaries of Bathinda rats. Estradiol and progesterone were also low in all rats collected from Bathinda regions. Chlorpyrifos residues levels were detected in the blood of rats collected from Bathinda district of Punjab. Histological investigation further revealed higher number of atretic follicles in all the stages of follicles in Tatera indica and Bandicota bengalensis rats collected from Bathinda region. The average diameter of all growing follicles were also less in the ovaries of Bathinda rats at P<0.05. From present studies it can be inferred that the alteration in biochemical constituents of ovary and may be due to the reduced synthesis of steroids in the female rats inhabiting Bathinda district of Punjab that can be attributed to pesticides evidenced by their presence in pesticide residue analysis.
Keywords | Enzyme, Follicles, Lipids, Malwa region, Ovary
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 28, 2018; Accepted | August 31, 2018; Published | September 26, 2018
*Correspondence | Parkash Singh. Department of Medical and Biotechnology, Baba Farid College, Bathinda-151001, India; Email: [email protected]
Citation | Singh P, Sangha GK (2018). Histomorphological and biochemical studies in ovaries of female tatera indica and bandicota bengalensis inhabiting south-west region of punjab in north india. Adv. Anim. Vet. Sci. 6(11): 480-485.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.11.480.485
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Singh and Sangha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The development of modern technology and the rapid industrialization are among the foremost factor for environmental pollution. The environmental pollutants are spread through different channels many of which finally enter in to the food chain of livestock and man (Kaplan et al., 2010). Pesticides having metals and other agrochemicals are some of the major causes of environmental toxicity in farm animals (Rajaganapathy et al., 2011).The wide spread use of these chemicals in agriculture has caused environmental pollution and potential health hazards (Agarwal and Sharma, 2010). Several pesticides and environmental contaminants are potential endocrine disrupters and female reproductive system may be regarded as sensitive target for theses disrupters (Bretveld et al., 2006; Agarwal and Sharma, 2010). They can alter development of reproductive system as well as ovulation and implantation (Stamati et al., 2007, Gopinath, 2013).
The Malwa region of Punjab India is facing an unprecedented crisis of environmental health linked to indiscriminate, excessive and unsafe use of pesticides, fertilizers and poor ground water quality. Studies of this region have also highlighted a sharp increase in many other pesticide-related diseases, such as mental retardation and reproductive disorders (Mittal et al., 2014). The increase in genetic damage in animals indicates the potential genetic hazards posed by excessive use of pesticides in Bathinda district and the long-time over-use of pesticides appears to be a major cause for prevalence of various diseases in cotton cultivated districts of the Malwa region of Punjab (Kalra and Sangha, 2018). The high use of pesticides along with environmental and social factors is responsible for the high concentration of pesticide residues in the food chain of this region (Mittal et al., 2014). Earlier studies give some indication of increased reproductive risks of exposure to pesticides/heavy metals but the epidemiological evidences do not allow any clear inference to be drawn (Thakur et al., 2008, Singh et al., 2012, Mittal et al., 2014). The present study was designed to examine the possible effect of environmental contaminants on the female fertility indices and ovarian functions in Tatera indica and Bandicota bengalensis inhabiting South-West region of Punjab.
Material and Methods
During the study, the field rats i.e. Tatera indica and Bandicota bengalensis were collected from Bathinda district of South-West region of Punjab using multi catch rat traps. The same species of rats were collected from Punjab Agricultural University, Ludhiana and adjoining areas as they served as control rats (as in these areas the spray of pesticides is in the permissible limit). Approval of Institutional Animal Ethical Committee, Guru Angad Dev Veterinary and Animal Science University (GADVASU) Ludhiana was obtained for the usage of animals vide letter No. 3901-35 dated 06.08.2012. Animals were brought to laboratory and separated according to age and sex.
Organ Weight and Organosomatic Index
Following humane sacrifice, the reproductive organs viz. ovary, oviduct, vagina and uterus were excised cleaned of the adhering tissue and weighed separately. The organo-somatic index (OSI) was calculated by using the following formula as per Chattopadhya et al. (2011).
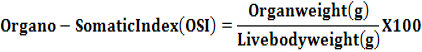
Biochemical Studies
For biochemical studies, one whole ovary was homogenized in 2 ml of phosphate buffer saline (PBS 0.1M, pH 7.4) and ovary homogenate was centrifuged at 3000 for r.p.m. for 10 min. The Supernatant was used for estimation of total soluble proteins and various enzymes. Protein was estimated by the method of Lowry et al. (1951). Acid phosphates (ACP) and alkaline phosphates (ALP) activity was measured in citrate buffer (0.05M pH 10.5) and glycine buffer (0.05M pH 10.5), respectively using p-nitrophenol phosphate as substrate following the method of Bessay et al. (1946). Total lipids from the ovaries tissue were extracted by the method of Folch et al. (1957). Total phospholipids were estimated by Ames, (1966) method and cholesterol was estimated by Chiamori and Henry, (1959) method from the above extracted lipids.
Hormone Assay
Blood sample from each rat was collected directly from heart by heparinised syringe in heparinised vials. Blood was centrifuged at 2300 r.p.m. for 15 minutes. Supernatant was obtained as plasma which was used for the determination of estrogen and progesterone concentration using ELISA kits.
Histological Studies
For histomorphological studies, ovary of Tatera indica and Bandicota bengalensis rats collected from Bathinda region and Ludhiana rats were fixed in alcoholic bouin’s solution for 24 hours. After complete fixation, the tissue was dehydrated in graded series of ethanol, cleared in xylene and embedded in paraffin wax (Melting point between 58-60°C). The 3-5µm thick sections were cut serially with the help of microtome and after usual de-waxing and rehydration in descending series of ethanol to water, the sections were stained in haematoxylin,- eosin, cleared in xylene and mounted in DPX for microscopic examination. All serial sections of the ovary were studied for various stages of development of follicles, total number of normal, atretic follicles, diameter of follicles and oocyte as described by Kaur and Guraya, (1983).
Statistical Analysis
Results were expressed as Mean ± standard error of mean (SEM) and subjected to student t-test. Results were considered statistically significant with p<0.05.
ResultS
There was significant decrease in the weight of ovary in Tatera indica and weight of vagina in female Tatera indica and Bandicota bengalensis Bathinda rats as compared to the Ludhiana rats (Table 1).The amount of total proteins was less in the ovaries of Tatera indica and Bandicota bengalensis female rats inhabiting Bathinda region of Punjab as compared to Ludhiana rats (Table 2). The level of various ovarian enzymes like acid phosphates (ACP) and alkaline phosphates (ALP) increased significantly in Tatera indica and Bandicota bengalensis Bathinda rats as compared to Ludhiana rats (Table 2). Hormonal balance is important to preserve female reproduction and maintain fertility. Changing level of estrogen or progesterone can disturb this balance. In the present study estrogen and progesterone level decreased in all Tatera indica and Bandicota bengalensis rats collected from Bathinda rats as compared to Ludhiana rats (Table 3).
Table 1: Relative weight of reproductive organs (g/100 g bw) in Tatera indica and Bandicota bengalensis female rats.
| T. indica | B. bengalensis | |||
| Organs | Ludhiana (Control) | Bathinda | Ludhiana (Control) | Bathinda |
| Ovary | 0.030±0.001 | 0.021±0.001* | 0.025±0.001 | 0.021±0.002 |
| Oviduct | 0.009±0.000 | 0.008±0.000 | 0.009±0.000 | 0.010±0.000 |
| Uterus | 0.096±0.003 | 0.090±0.006 | 0.094±0.003 | 0.100±0.005 |
| Vagina | 0.104±0.002 | 0.082±0.003* | 0.108±0.002 |
0.088±0.002* |
Values are Mean ± SE,
*Significant difference at (p<0.05) as compared to control
Table 2: Levels of Proteins, Total lipids, Cholesterol and Phospholipids (mg/g wet wt. of ovary) and activity of acid and alkaline phosphatase (ICU) in ovaries of female Tatera indica and Bandicota bengalensis.
| T. indica | B. bengalensis | |||
| Biochemical constituents | Ludhiana (Control) | Bathinda | Ludhiana (Control) | Bathinda |
| Proteins | 8.054±0.546 | 6.802±0.291 | 8.215±0.855 | 7.946±0.245 |
| Total lipids | 14.25±1.200 | 13.65±0.854 | 19.20±0.145 | 14.85±0.478* |
| Cholesterol | 9.180±0.865 | 5.905±0.435* | 9.451±1.354 | 6.888±0.421* |
| Phospholipids | 10.206±1.081 | 7.763±0.409* | 10.486±1.610 | 9.153±0.874 |
| ACP | 3.476±0.434 | 5.380±0.493* | 3.939±0.481 | 4.708±0.707 |
| ALP | 14.124±1.172 | 16.402±1.056 | 15.551±2.348 |
17.069±1.270 |
Values are Mean ± SE,
*Significant difference at (p<0.05) as compared to control
Table 3: Progesterone and Estrogen concentration (pg/ml) in ovaries of female Tatera indica and Bandicota bengalensis.
|
Hormones |
T. indica | B. bengalensis | ||
| Ludhiana (Control) | Bathinda | Ludhiana (Control) | Bathinda | |
| Estrogen | 370.25±15.07 | 411.00±12.32 | 359.10±18.00 | 268.01±8.24 |
| Progesterone | 327.53±11.24 | 294.22±9.02 | 261.24±21.64 | 189.33±14.15 |
Values are Mean ± SE,
*Significant difference at (p<0.05) as compared to control
Table 4: Percentage of atresia in ovaries of female Tatera indica and Bandicota bengalensis.
| Species | Area | Condition of Ovary | Number of primary follicles | Number of secondary follicles | Number of tertiary follicles | Number of pre antral follicles | Number of antral follicles | Number of Corpus Luteum |
|
Tatera indica |
Ludhiana (Control) | Normal | 26.22±1.34 | 11.00±1.02 | 3.00±0.02 | 11.05±3.22 | 6.64±0.22 | 4.00±1.00 |
| Atretic | 9.25±0.028 | 3.00±0.24 | 1.02±0.03 | 1.33±0.88 | 2.22±0.88 | 1.22±0.02 | ||
| % Atresia | 26 | 21.4 | 25.5 | 10.7 | 25 |
23.3 |
||
| Bathinda | Normal | 18.22±2.10 | 9.00±2.22 | 3.24±0.07 | 7.02±1.00 | 4.02±0.25 | 2.00±0.25 | |
| Atretic | 5.00±0.12 | 3.22±0.42 | 1.44±0.33 | 4.22±0.24 | 2.00±0.15 | ----- | ||
| % Atresia | 21.53 | 26.3 | 30.7 | 37.5 | 33.2 | ----- | ||
|
Bandicota bengalensis |
Ludhiana (Control) | Normal | 23.25±2.34 | 9.00±1.24 | 4.24±0.425 | 12.24±2.25 | 6.24±0.20 | 5.25±1.02 |
| Atretic | 11.00±1.24 | 4.02±0.22 | 2.00±0.01 | 5.24±1.02 | 2.45±0.25 | 1.00±.025 | ||
| % Atresia | 32.1 | 30.17 | 32.05 | 29.9 | 28.19 | 16.00 | ||
| Bathinda | Normal | 18.00±2.45 | 11.84±2.63 | 3.55±0.34 | 10.00±2.04 | 4.25±1.25 | 4.25±1.00 | |
| Atretic | 7.45±1.22 | 5.26±1.00 | 1.96±0.22 | 6.25±0.86 | 2.96±.45 | 2.00±0.425 | ||
| % Atresia | 29.27 | 30.76 | 35.57 | 38.46 | 41.05 |
32.00 |
Values are Mean ± SE,
*Significant difference at (p<0.05) as compared to control
Table 5: Diameter of follicles (µm) in ovaries of female Tatera indica and Bandicota bengalensis.
| Tatera indica | Bandicota bengalensis | |||
| Category of follicles | Ludhiana (Control) | Bathinda | Ludhiana (Control) | Bathinda |
| Primary |
111.40±21.15 (32.5-147.5) |
105.00±21.86 (32.5-150) |
109.44±19.06 (45-123.55) |
99.44±18.52 (35.2-147) |
| Secondary |
116.90±21.70 (75-180) |
114.50±14.92 (80-150) |
116.2±9.27 (90.25-140.25) |
106.20±13.41 (78.5-150.5) |
| Teritary |
133.40±5.56 (111.5-140.25) |
139.50±6.79 (127.5-162.5) |
139.2±15.63 (111-180.25) |
139.25±11.54 (111-175) |
| Preantral |
174.62±9.91 (158.25-205.25) |
178.50±9.162 (172.5-202.5) |
187.64±12.88 (150-180.5) |
187.64±4.59 (175.2-198.5) |
| Antral |
167.05±14.97 (140-223.25) |
190.00±11.70 (152.5-222.5) |
200.50±9.78 (150.25-230) |
200.51±12.94 (160.5-229) |
Values are Mean ± SE,
*Significant difference at (p<0.05) as compared to control; Figures in parenthesis indicates the range of diameter
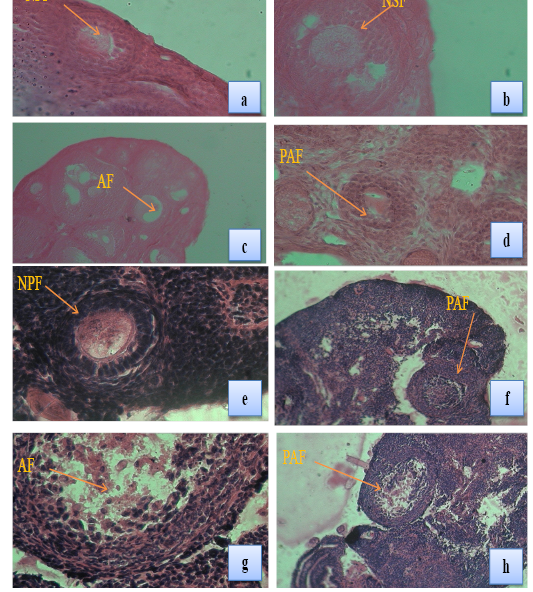
Plate 1: Fig a & b: T.S. of ovary of a control female T. indica showing normal primary follicle (NPF) & (NSF) (X100) & (X 400); Fig c & d: T.S. of ovary of a female T. indica collected from Bathinda district showing atretic follicle (AF) in the ovary (arrow), primary atretic follicle (PAF) and early antral atretic follicle (EAAF) (X100) & (X 400); Fig e: T.S. of ovary of a control female B. bengalensis showing normal primary follicle (NPF) (X100); Fig f, g & h: T.S. of ovary of a female B. bengalensis collected from Bathinda district showing atretic follicle in the ovary (arrow), primary atretic follicle (PAF) and early antral atretic follicle (EAAF) (X100) & (X 400).
The present study revealed that the number of normal follicles in all the stages of follicles was higher in Ludhiana Tatera indica and Bandicota bengalensis and their number decreased in the ovaries of rats collected from Bathinda region (Table 4; Plate 1 Fig a-h). The average diameter of all growing follicles was also less in the ovaries of Bathinda rats than PAU control rats (Table 5).
Discussion
The loss of organ weight in ovaries was probably due to alteration in reproductive hormones (Liu et al., 2006). The decrease in weight and size of ovaries can be due to extensive fibrosis and atretic follicles (Baligar and Kaliwal, 2002; Gopinath, 2013). Organophosphates like chlorpyrifos, diazinon, methyl parathion, dimethoate and monocrotophos given to female albino rats have also resulted in significant decrease in the ovarian weights (Kaur and Dhanju, 2005; Johari et al., 2010; Nishi and Hundal, 2013).
The decrease in the level of proteins in Bathinda rats as compared to the control rats may indicate the induced degenerative changes in the ovaries of rats or general disturbance of the proteins anabolism which may be due to androgen/estrogen deficiency leading to impaired game to genesis (Bretveld et al., 2006). Low levels of lipids, phospholipids and cholesterol in these rats indicated decreased steroidogenic activity in atretic follicles and degenerating corpora lutea and are due to loss of intracellular membranes in degenerating tissues (Wellington et al., 2004). Organophosphates like methyl parathion, dimethoate and monocrotophos when given to female albino rats at a dose level of 1/5th of LD50 had also resulted in significant decrease in concentration of proteins, lipids, phospholipids and cholesterol in ovaries of treated rats (Kaur and Dhanju, 2005). Shakoori et al. (1992) also reported a significant reduction in cholesterol levels in rats treated with cypermethrin. Phosphatases are involved in many different processes that require mobilization of phosphate ions or dephosphorylation as part of anabolic, catabolic or transfer processes (Kaur and Dhanju, 2004). A change in enzyme activity is generally related to intensity of cellular damage (Muthuviveganandavel et al., 2008). The peroxidation of membrane phospholipids not only alters the lipid milieu and structural and functional integrity of cell membrane, but also affects the activities of various membrane bound enzymes including ATPases (Rhodes et al., 1984).
It has been reported that the insecticides may destroy endocrinologic homeostasis by suppressing Gn-RH release, may act directly on the gonadotropins to alter the gonadotropin synthesis and secretion or indirectly by altering the pituitary cell responsiveness to GnRH or gonadal steroids which result in the alterations in the levels of FSH and LH affecting the feed-back mechanisms (Stoker et al., 1993). Earlier studies have demonstrated that various pesticides cause a decrease in circulating estradiol and progesterone levels in rats, monkeys and human beings and result in the endocrinal dysfunction (Bretveld et al., 2006; Johari et al., 2010). Evidences have also suggested that organochlorine pesticides, even at low concentrations, may disrupt the endocrine system which was responsible for proper hormone balance (Mantovani, 2002; Figa-Talamanca et al., 2001).
The quantitative assessment of follicle number is an indicator of the normal function as well as toxic responses in the ovary (Plowchalk et al., 1993). Earlier studies have also reported reduction of different types of healthy follicular stages with concomitant increase in atresia in rats and mice treated with different pesticides (Baligar and Kaliwal, 2002; Koc et al., 2009). Ataya et al. (1988) have reported that the cyclophosphamide is found to inhibit the development of the antral follicles in rats, thereby increasing atretic follicles in rats through interfering with hormonal ovarian follicular development and reduces estradiol. It has also been shown that the ovarian androgen and inhibin secretion by follicles may be an important part in the regulation of FSH secretion and follicular growth (Evans et al., 1997). A wide variety of environmental agents may be responsible for modifying the neurotransmitter levels that would act at the level of hypothalamus to adversely affect the reproductive functions. Organophosphates like chlorpyrifos, methyl parathion, dimethoate and monocrotophos given to female albino rats have also resulted in increased atresia of follicles in treated rats as compared to control rats (Kaur and Dhanju, 2005; Nishi and Hundal, 2013). The increase in antral follicles artesia and ovarian epithetlial proliferation may be either by directly targeting the tissues or indirectly via alteration of endogenous hormones (Borgest et al., 2002; Bretveld et al., 2006; Nishi and Hundal, 2013). The increase in genetic damage in rats indicates the potential genetic hazards posed by excessive use of pesticides in Bathinda district. The long-time over-use of pesticides appears to be a major cause for prevalence of various diseases in Malwa region of Punjab (Kalra and Sangha, 2018).
Conclusion
The alteration in biochemical constituents of ovary and decrease in ovarian weight, number of healthy follicles may be due to the reduced synthesis of steroids in the Tatera indica and Bandicota bengalensis female rats inhabiting Bathinda district of Punjab that can be attributed to pesticides evidenced by their presence in pesticide residue analysis.
Acknowledgements
The author is very thankful to the UGC that awarded Rajiv Gandhi National Fellowship (RGNF) for the financial support, head department of Zoology, Punjab Agricultural University Ludhiana and Punjab for providing necessary facilities to carry out the research work.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Author’s contribution
Both the authors made substantial contributions to the design, acquisition of data, analysis and interpretation of data; Authors participated in drafting the article or revising it critically for important intellectual content; and gave final approval of the version to be submitted and any revised version.
References






