Advances in Animal and Veterinary Sciences
Research Article
Morphological and Histological Study of the Cecum and Colon in Adult Local Camelus dromedarius
Ahmed Adeeb Mohamed1*, Khalid Hadi Kadhim2, Diyar Mohammad Hussein2
1College of pharmacy, AL Muthanna University, Samawa, Iraq, 66001; 2Department of Anatomy and Histology, College of Veterinary Medicine, Al Muthanna University, Samawa, Iraq, 66001.
Abstract | The present study was designed to describethe morphological and histological structures of the cecum and colon in adult local one humped camel in Iraq. Specimens of cecum and colon were collected from five healthy male camels between 4-5 years old. The samples were collected immediately after slaughtering and transferred to the Laboratory of Anatomy, College of Veterinary Medicine for further processing. For gross study, the intestinal tract was separated and dissecting it away from it attachments to the dorsal abdomen wall. The cecum appeared as smooth cylindrical sac and its measuring were (51.35± 6.40) cm. (72.5± 9.4) gm. (0.124 ± 0.07) cm for mean total length, weight(weight after empty of the part) and wall thickness respectively. The colon was appearedas one long continuous hollow tube with externally smooth and divided into ascending, transverse and descending parts. Histologically, the cecum and colon wall was composed of the four tunicae (mucosa, submucosa, muscularis and serosa or adventitia).The tunica mucosa was the inner layer lined of the cecum and colon lumen. The mean thickness of these tunica in colon were more than that in cecum (487.9±26.6)μm, (366.7±34.1)μm respectively. The tunica mucosa revealed three different layers including: lining epithelium, lamina propria with glands and muscularis mucosa. The colon epithelium revealed large number of Lieberkuhncrypts and goblet cells reached (31 ± 4), (262 ± 11) respectively, more than that in cecum (27 ± 5), (218 ± 12), respectively. The submucosa consists of irregular connective tissue and adipose tissue, numerous blood vessels. The mean thickness of these tunica in cecum was more than that in colon (243.2±44.3)μm and (124.8±16.6)μm respectively. Tunica muscularis was composed of inner circular and outer longitudinal smooth muscle layers with mean thickness of these tunica in colon was more than that in cecum (694.7±37.4)μm and (513.9±46.3)μm respectively. The tunica serosa or adventitia was consist of loose connective tissues with mean thickness of these tunica in colon was more than that in cecum (212.7±14.1)μm and (187.9±17.6)μm respectively. In conclusion, this study identified the morphological and histological features of adult Camelus dromedaries colon and cecum.
Keywords | Camelus dromedaries, Cecum, Colon, Morphology, Histology
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 12, 2018; Accepted | June 27, 2018; Published | July 25, 2018
*Correspondence | Ahmed Adeeb Mohamed, College of pharmacy, AL Muthanna University, Samawa, Iraq, 66001; Email: [email protected]
Citation | Mohamed AA, Kadhim KH, Hussein DM (2018). Morphological and histological study of the cecum and colon in adult local camelus dromedarius. Adv. Anim. Vet. Sci. 6(7): 286-291.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.7.286.291
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Camels belong to the taxonomic order Artiodactyla (even toed Ungulates), sub-order Tylopoda (Pad-footed), of the family Camelidae. The Camelidae is a relatively small family of two genera: Camelus (Old world camels) and Lama (New world camels). The genus Camelus consists of Camelus dromedarius, commonly known as the dromedary, one-humped or Arabian camels and Camelus bactrianus, the Bactrian or two-humped camels (Burton et al., 1969; Burton, 1972; Wilson, 1984). The one-humped Dromedary is the largest mammalianspecies. It is adapted to the desert where thorny plants with rough and hard stems grow and with its high temperatures and extreme desiccation (Bello et al., 2012). Dromedary camel is widely distributed in the desert of the Arabian countries. It is characterized by its ability to tolerate greater than 30% water loss, which is generally impossible for other mammals (Rizk et al., 2017). One- humped Camel is an important multi-purpose animal in arid and semi-arid areas of the world. There are about 20 million camels in the world (FAO, 1992). They are kept for a variety of purposes e.g. transportation, racing (Osuebeni et al., 1999), and as source of human food (Dorman, 1986). The digestive anatomy and physiology of dromedarian camel at embryonic level is least understood when compared to Llama, Guanaco, Cattle, Sheep, Goat and Pig (Cummings et al., 1972; Bustinza, 1979). The description of dromedarian camel is usually made as if it is identical with Llama species (Cummings et al., 1972).Though, they are pseudo-ruminants that possess a three-chambered stomach, lacking the omasum that is part of the four-chambered stomach of the order Ruminantia (Bustinza, 1979; Wilson, 1995). The true camels (Camelus dromedarius and Camelus bacterianus) are closely related anatomically to the South American Camelids (Franco et al., 2004).The large intestine consists of different parts (segments) with various functions. The segments of these parts are: the transverse colon, ascending colon, appendix (when present), descending colon, rectum and anus (Dyce et al., 2010). In the process, fecal balls are formed, which can be passed through the rectum and are expelled out the anus (Cummings et al., 1972). Review of literature revealed scarce information concerning colon and cecum micro and macro anatomy of local Iraqi camel. Consequently, this study designed to describe the gross and microscopic features of the cecum and colon of the one-humped Dromedary (Camelus dromedarius).
Materials and methods
Specimens were collected from five healthy local one-humped camel males (Camelus dromedarius), between 4 to 5 years old that slaughtered at AL-Muthanna abattoir. All collected specimens were free from gross pathological changes. For gross study the intestinal tract was separated and dissecting it away from it attachments to the dorsal abdominal wall, the total length, thickness of wall and weight of the cecum and colon (weight after empty of the parts) (Perez, 2016), measured by digital electronic verinia, measurement tape and ruler. The cecum was taken from the apex border to the caeco-colic junction. The length of the colon was taken from the ileo-caeco-colic junction to the beginning of the sacculation at colo-rectal junction. For histological study one centimeter of different segments of cecum and the colon (proximal anza, centripetal, centrifugal, and distal anza) were fixed in 10% formalin for 48 hours at room temperature. The tissues were processed following standard histological procedure (dehydration with an ascending grade of alcohol, clearing in xylene and impregnation (embedding) in liquid paraffin wax) was performed. Immediately after embedding, the specimens were processed for histology using standard histological techniques. The tissues were sectioned at 5 μm thickness and stained with hematoxylin and eosin (H&E) (Luna, 1968). The slides were then dipped in xylene and mounted with cover slip using DPX mounting medium. The slides were examined under light microscope and images were captured by connected digital camera. The following layers were examined and measured:
1- Thickness of the tunica mucosa (epithelium, lamina propria and muscularis mucosa), tunica submucosa, tunica muscularis (circular and longitudinal layers) and tunica serosa.
2- Crypts of Lieberkuhn and goblet cells counts, ten sections from each part, in each section the crypts and cells in ten microscopic fields were counted. (Longitudinal sections used only).
The mean (X-) and the standard error (S.E) were calculated for 5 slides for each part of the examined tissue (Al-Rawi and Kalaf-Allah, 1980).
Results
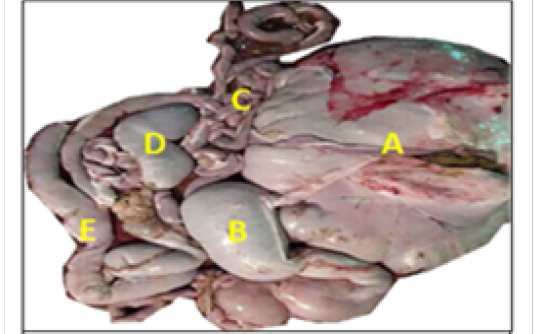
The cecum was appeared as a blind-ending piece of gut that arises at the junction of the ileum and colon and attached to mesentery at the right side of abdomen (Figure 1). Thececum mean length, weight after removed of the contents and mesenteriesand thickness of wall were (51.35± 6.40) cm, (72.5± 9.4) gm and (0.124 ± 0.07) cm respectively (Table 1). The colon appeared as one long continuous hollow tube (Figure 1), and its mean length, weight after removed of the contents and mesenteries and wall thickness were (642.44±34.12) cm. (863.6±32.4)gm and (0.158±0.06) cm respectively (Table 1).
Table 1: Measurement of length(cm), weight(gm) and thickness of the wall(cm), of the cecum and colon (X± S.E).
| Measure/Part | Length (cm) | Weight(gm) | Thickness(cm) |
| Cecum | 51.35 ± 6.40 | 72.5 ± 9.4 | 0.124 ± 0.07 |
| Colon | 642.44 ±34.12 | 863.6 ± 32.4 | 0.158 ± 0.06 |
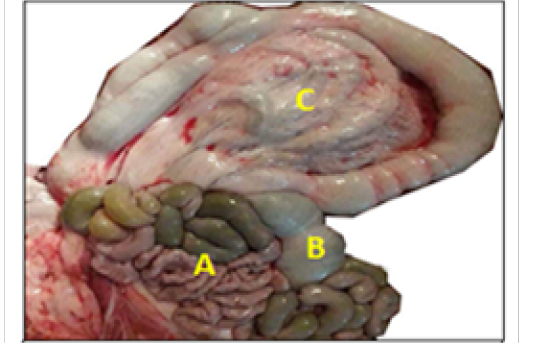
Macroscopically, the colon was divided into ascending, transverse and descending parts. The ascending colon had three ansa: the proximal ansa, the spiral ansa and the distal ansa. The ascending colon was the most developed portion of the colon and located in the abdomen left side (Figure 2).
Table 2: Measurement of thickness of the wall layers of the cecum and colon (µm) (X± S.E).
| Measure /Part | Mucosa | Submucosa | Muscularisexternia | Serosa or Adventatia |
| Cecum |
366.7± 34.1 |
243.2± 44.3 |
513.9± 46.3 |
187.9± 17.6 |
| Colon |
487.9± 26.6 |
124.8± 16.6 |
694.7± 37.4 |
212.7 ± 14.1 |
The cecum and colon wall was composed of the four tunicae (mucosa, submucosa, muscularis externa and serosa or adventitia).The tunica mucosa or the inner layer lined of the cecum and colon lumenwithout villi or plicae circulares. Colon mucosa and the submucosa exhibited temporary folds (Figure 3 and 4). The mean thickness of these tunica in colon was more than that in cecum (487.9±26.6) μm and (366.7 ± 34.1) μm respectively (Table 2). The tunica mucosa were included three different layers; the lining simple columnar epithelium, the lamina proparia composed of loose connective tissue and several crypts of Lieberkuhn; and smooth muscle of the muscularis mucosa (Figure 5 and 6). The colon epithelium has large number of goblet cells. Goblet cells appeared as globular shaped unicellular mucous that dispersed among the columnar cells of the epithelium and lined of crypts of Lieberkuhn in tunica mucosa of the whole cecum and colon (Figure 5, 6 and 7).
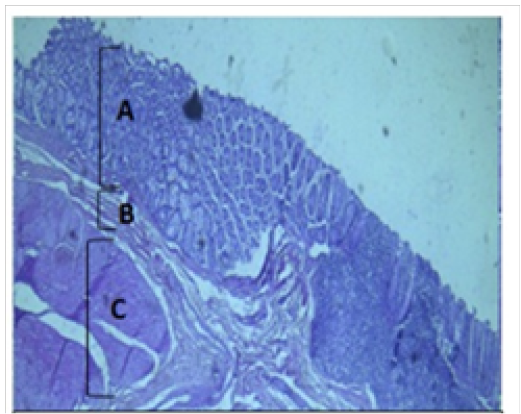
Figure 3: Histological section of cecum Showing: A- Mucosa B- Submucosa C- Muscularis Externia H&E 40X
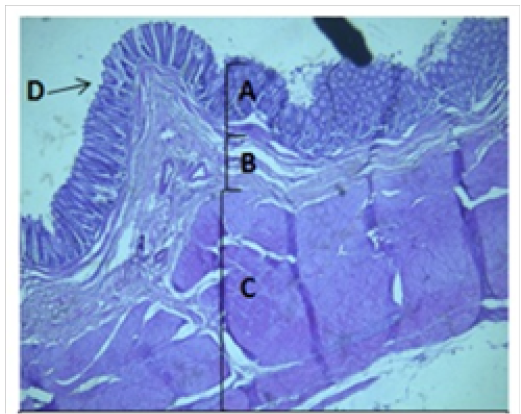
Figure 4: Histological section of colon Showing: A- Mucosa B- Submucosa C- Muscularis Externia D- Folded H&E 40X
The colon revealed large number of crypts of Lieberkuhn and goblet cells (31 ± 4) and (262 ± 11) respectively more than that in cecum (27 ± 5) and (218 ± 12) respectively (Table 3). The cecum and colon submucosa showed dense connective tissue that revealed absence of glands and aggregates of lymphatic nodules (Figure 5). The mean thickness of cecum submucosa was more than that in colon (243.2±44.3)μm and (124.8±16.6)μm respectively (Table. 2). The smooth muscle layers of muscularis externia revealed inner circular muscle layer in colon, however it wasrandomly oriented in cecum and its modified thickenings of the outer longitudinal muscle of the colon called taenia
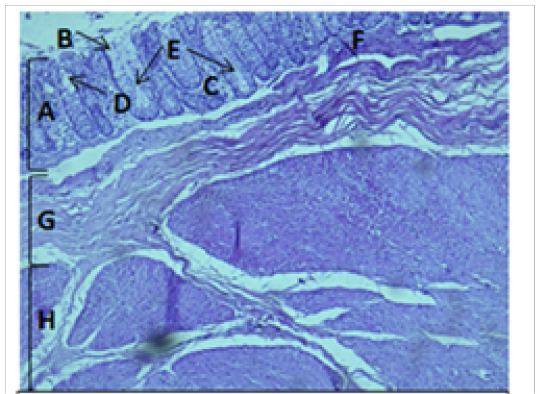
Figure 5: Histological section of cecum Showing: A- Mucosa B- Epithelium C- Lamina proparia D- Goblet cell E- Crypts of lieberkuhn F- Muscularis mucosa G- Submucosa H- Muscularis Externia H&E 100X
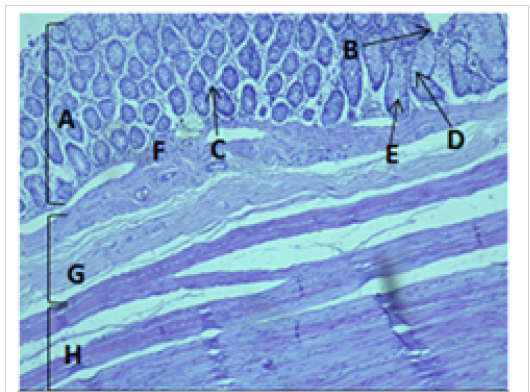
Figure 6: Histological section of colon Showing: A- Mucosa B- Epithelium C- Lamina proparia D- Goblet cell E- Crypts of lieberkuhn F- Muscularis mucosa G- Submucosa H- Muscularis Externia H&E 100X
coli. The mean thickness of these tunica in colon was more than that in cecum (694.7±37.4)μm and (513.9±46.3)μm respectively. The colon and cecum revealed also both adventitia and serosa loose connective tissues with mesothelium, the mean thickness of these tunica in colon was more than that in cecum (212.7±14.1)μm and (187.9±17.6)μm respectively.
Table 3: The number of crypts of Lieberkuhn and goblet cells per microscopic field (40X) in cecum and colon (µm) (X± S.E).
| Measure/Part | Crypts of Lieberkuhn | Goblet cells |
| Cecum | 27 ± 5 | 218 ± 12 |
| Colon | 31 ± 4 | 262 ± 11 |
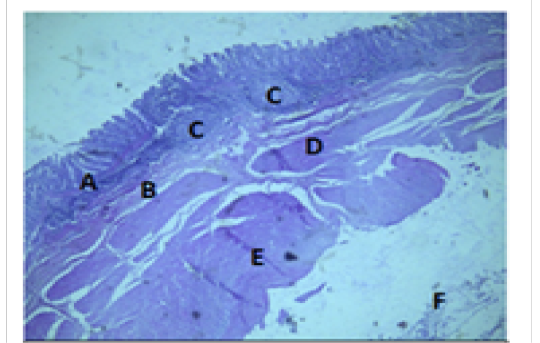
Figure 7: Histologiccal section of cecum Showing: A- Mucosa B- Submucosa C- Lymphatic nodules D- Inner layer of muscularis Externia E- Outer longitudinal layer of muscularis Externia F- Serosa H&E 40X
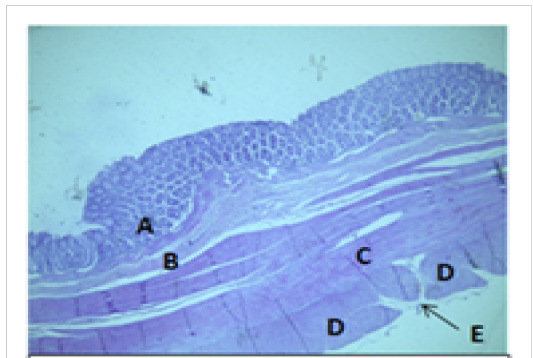
Figure 8: Histological section of colon Showing: A- Mucosa B- Submucosa C- Inner circular layer of muscularis Externia D- Outer longitudinal layer of muscularis Externia (taenia coli) E- Serosa H&E 40X
Discussion
Digestive system of camelids is somehow alike to ruminants in several aspects, including regurgitation of ingesta and active microbial fermentation in the stomach (Frandsonet al., 2003). The small and large intestine of the Llama and the Alpaca occupy most of the space caudally and caudodorsal to the abomasum, particularly in the right paralumbar fossa (Cebra et al., 2002). However, it lies almost entirely to the right of the midline, packed mainly into the dorsal part of the abdomen, partially in the hypochondriac sub region, in ruminants (Dyce et al., 2010). Both caecum and colon were smooth externally and had no sacculations or bands. Colon is larger in its part and coiled in manner similar to that of the pig (Dyce et al., 2010).The gross anatomy of this study showed that ascending colon of the camel had three ansae (proximal, spiral and distal) and this results are in agreement with observations described previously for bovine, ovine and caprine (Franco et al., 1993) and Myocastor coypus (Perez et al., 2008). The main function of the cecum and colon as parts of the large intestine is to reclaim excess moisture and return it to the body (Franco et al., 1993). Fecal balls are formed and passed through the rectum and are expelled out the anus (Cumming,1972). One of the functions of the colon is to absorption of vitamins B and K, some electrolytes (Na+ and Cl−), and most of the remaining water, to form stools, and to eliminate it (Maloiy et al., 1980). The proximal half of the colon absorbs salts (e.g., sodium chloride), water, and vitamins produced by bacteria. Storage, however the distal half of the colon holds feces until it is eliminated (Pérez et al., 2016).
In the present study the cecum appeared as a blind-ending piece of gut that arises at the junction of the ileum and colon. Moreover, its attached to mesentery and the colon was structured as one long continuous hollow tube. Simultaneously, the colon was divided into ascending, transverse and descending parts. The ascending colon had three ansa: the proximal ansa, the spiral ansa and the distal ansa. The ascending colon was the most developed portion of the colon that located in left side of abdomen. These observations arein agreement with previous reported findings in the alpaca, dromedary and Giraffe (Pérez et al., 2016; Pérez et al., 2009).
The cecum and colon wall was composed of the four tunicae (mucosa, submucosa, muscularis externa and serosa or adventitia). The tunica mucosa is the inner layer that lined the cecum and colon lumen and didn’t revealed villi. This result is in agreement with observations reported previously in ruminants and camelid (Dellmann and Brown, 1987; Althnaian et al., 2013). The tunica mucosa of cecum and colon was included three different layers;the lining simple columnar epithelium with large number of goblet cells, the lamina proparia composed of loose connective tissue and several crypts of Lieberkuhn and smooth muscle of the muscularis mucosa. However, the submucosa of cecum and colon revealed dense connective tissue and no gland and aggregates of lymphatic nodules extending to the mucosa. The smooth muscle layers of muscularis externia revealed inner circular muscle layer in colon and were slightlyand randomly oriented in cecum with modified thickenings of the outer longitudinal muscle of the colon that called taenia coli. These findings are compatible with previous observations reported for the one humped camel colon (Al Hussany et al., 2013).
The lining epithelium of the entire colon is simple columnar with large number of goblet cells. The glands appeared as simple straight tubular with large number of goblet cells and thin muscularis mucosa that was always present below the glands separating the lamina propria from the tunica submucosa. In few regions of the tunica submucosa, a small aggregation of lymphoid tissues was observed. The tunica submucosa was composed of regular connective tissue with blood vessels, and lymphatics scattered within it. Submucosal nerve plexus and ganglia were present inside the tunica submucosa. The tunica muscularis appeared to consist of relatively thick inner circular layer and much smaller outer longitudinal cell layers. Inner circular layer appeared to consist of two layers of almost equal sizes separated by thin intermuscular connective tissue and the cecum of the camel likely cecum of the Giraffe, which was smooth externally and had no sacculations or bands (Perez et al., 2009). These observations are unlikely to previous reported results of equine cecum and in cattle, sheep and goat (Kadam et al., 2011). In conclusion, this study described the macro and microscopical observations on the camel and cecum and colon. The authors recommend more studies on the different parts of the camelids digestive system and compare it with others ruminants.
Acknowledgements
The authors gratefully acknowledge college of veterinary medicine / the university of AL Muthanna for the their gracious assistance.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authors Contribution
All authors contributed equally.
References