Advances in Animal and Veterinary Sciences
Research Article
Changes in the Relative Abundance of miR-205, miR-26a-5p, let-7b and their Target Genes in Vitrified Bovine Embryos after Phenazine Ethosulfate Supplementation
Yasser H.A. Saber1, Sally Ibrahim1, Karima Gh. M. Mahmoud1*, Wahid M. Ahmed1, Refaat S.A. Ragab2, Adel A.M. Seida2*
1Department of Animal Reproduction and A.I, Veterinary Research Division, National Research Centre, Dokki, 12622 Giza, Egypt; 2Department of Theriogenology, Faculty of Veterinary Medicine,Cairo University, Giza, Egypt.
Abstract | Accumulation of lipid droplets is a main obstacle for cryopreservation and low survivability of embryos. This investigation aimed to study the effect of phenazine ethosulfate (PES) supplementation in culture media on the selected miRNAs (miR-205, miR-26a-5p, and let-7b) and their target genes (OCT4, DNMT, CASP3, ATF6, ATP5ME, and ELOVL5), during bovine embryo production. Therefore, a group of two-day bovine embryos was cultured in a medium with lipid-reducing agent, PES (0.3 mM). Another group of embryos without PES was served as a control. Embryos were vitrified and morphologically examined after warming. The viability also was evaluated by culturing for 24 h. After evaluation, embryos were classified into a good or a poor. Afterwards, embryos (blastocyst and morula) were kept at -80°C for RNA extraction and qRT-PCR of the selected miRNAs and their targets. Results revealed that the rate of morula was higher (P<0.01) in treated compared to control groups. After vitrifications, the percentage of good quality embryos increased in treated groups than control groups. Additionally, the rate of dead embryos was higher in control groups. The Let-7b and miR-205 were significantly over-expressed in the treated good as well as poor embryos compared to control (untreated) good and poor embryos, respectively. However, miR-26 was suppressed in the treated good and poor embryos compared to control (untreated) embryos, respectively. Both of OCT4 and DNMT1 transcripts up-regulated in the treated (good and poor) embryos compared to control groups. The ELOVL5 gene decreased in the treated (good and poor) embryos, compared to control ones. In conclusion, PES supplementation reduced lipid droplets, and improved cryotolerance of the blastocysts via modulation the expression pattern of miRNAs and their target genes.
Keywords | miRNA, Bovine, Phenazine ethosulfate, Vitrifications, Embryos quality
Received | July 01, 2021; Accepted | August 16, 2021; Published | November 01, 2021
*Correspondence | Karima Gh. M. Mahmoud, Adel AM. Seida, Department of Animal Reproduction and A.I, Veterinary Research Division, National Research Centre, Dokki, 12622 Giza, Egypt; Department of Theriogenology, Faculty of Veterinary Medicine,Cairo University, Giza, Egypt; Email: [email protected]; [email protected]
Citation | Saber YHA, Ibrahim S, Mahmoud KGM, Ahmed WM, Ragab RSA, Seida AAM (2021). Changes in the relative abundance of miR-205, miR-26a-5p, let-7b and their target genes in vitrified bovine embryos after phenazineethosulfate supplementation. Adv. Anim. Vet. Sci. 9(12): 2157-2167.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2157.2167
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Saber et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Many genetic companies that are concerned with artificial insemination field have started operating in embryo market, which has resulted in an increasing demand in in-vitro (IVP) embryos. This strategy resulted in a profound change in global cattle genetic market (Marsico et al., 2019). Although the great efforts have been done by researchers to improve IVP during the last years, the efficiency remains low (Sudano et al., 2019). The IVP embryos are less resistant to cryopreservation and revealed lower survivability compared with in vivo produced, because of increased lipid droplets (Sanches et al., 2017).
During embryonic development, the required energy is different from stage to another (Thompson, 2000). At the very early stage of embryonic development, embryos rely on oxidative phosphorylation to synthesize adenosine triphosphate since their metabolism is poor (Nelson and Cox, 2011). Then, the metabolism becomes higher and embryos use glycolysis process as well as amino acids for blastocele formation (Lima and Souza, 2009). In spite of lipids have a beneficial and a vital role in early embryonic development through providing energy. Their accumulations in the embryonic cytoplasm are reduced embryonic survivability following cryopreservation (Dias et al., 2017). Many studies were done to overcome IVP embryos’ low cryopreservation resistance using phenazine ethosulfate (PES) as delipidating chemicals agents (Barceló-Fimbres and Seidel, 2007; Sudano et al., 2011; Ghanem et al., 2014). These chemical agents reduce the fatty acids absorption and synthesis by cells (Batista et al., 2014; Dias et al., 2017).
It was found that PES inhibits fatty acids synthesis by NADH oxidation to NADP, which subsequently promotes the energy metabolism balance, as well as pentose phosphate pathway (PPP) reactions (Sudano et al., 2011; Ghanem et al., 2014). It was indicated that addition of 0.3 μM PES during in vitro culture (IVC) of bovine embryos accelerated glucose metabolism and reduced the amount of large lipid droplets in cytoplasm (De La Torre-sanchez et al., 2006). Furthermore, culture of bovine embryos with PES revealed obvious improvements in embryonic cryotolerance and survival rate after cryopreservation (Barceló-Fimbres and Seidel, 2007).
No doubt that the morphological assessment that used for evaluation embryo quality is not sufficient for the accurate judgment of embryonic viability. So, the precise assessment and selection of good quality embryos could be achieved by molecular investigation of certain markers that are relevant to each stage of embryonic development (Spricigo et al., 2014; Leme et al., 2016). Additionally, analysis of genes related to lipid metabolism is very important in order to understand the embryonic response to different treatments, which used to reduce lipid droplets in embryonic cytoplasm and improve cryotolerance as well as survivability (Baldoceda et al., 2015).
MicroRNAs (miRNAs) is a tiny non-coding RNAs; around 19–22 nucleotides in length (Ling et al., 2013). This small non-coding RNAs is one of key-player at post-transcriptional level, and express in oocytes, testis, granulosa cells, and embryos (Lin et al., 2019). It was found that miRNAs regulate many genes in stage specific manner, during different stages of embryonic developmental (Kropp and Khatib, 2015; Salilew-Wondim et al., 2020). Also, it was shown that embryos are well equipped with a huge amount of maternal miRNAs that inherited by embryos until activation of early embryonic genome (Tesfaye et al., 2018).
It was indicated that the octamer-binding transcription factor4 (OCT4) is one of the master regulators for pluripotency during early stages of embryonic development (Sadeesh et al., 2016). Besides, DNA methyltransferase (DNMT) plays an important function during oocytes maturation and early stages of embryonic development (Lodde et al., 2009). Michalak and Gye (2015) reported that activating transcription factor 6 (ATF6) is one of coping gene, which induced in preimplantation embryos as a normal part of the cellular adaptive mechanism to endoplasmic reticulum (ER) stress. Apoptosis-related cysteine peptidase (CASP3) has a critical role in the process of early apoptosis, and their up-regulation reflects early apoptosis (Wei et al., 2017). Genes such as: ATP5F1E (ATP synthase peripheral stalk-membrane subunit B) and ATP5ME (ATP synthase membrane subunit E) play important roles in the mitochondrial respiration, energy transport, and ATP synthesis during embryonic development (El-Sheikh et al., 2019). At early bovine embryo development, lipid fluctuation was observed with increase in ELOVL5 (ELOVL fatty acid elongase 5) expression, whereas ELOVL5 is responsible for fatty acids elongation at the morula stage (Sudano et al., 2016).
Despite the beneficial influences of PES on embryo quality that produced in vitro. The effect of PES was investigated only at the transcription level mainly for genes that regulate lipid metabolism and mitochondrial function. So far, the impact of PES on microRNA that fine-tune genes associated with lipid metabolism, mitochondrial function and viability as well as pluripotency genes was not study in bovine embryos. Therefore, the current study was aimed to investigate the effect of PES supplementation in culture media on the selected miRNAs (miR-205, miR-26a-5p, and let-7b) and their target genes (OCT4, DNMT, CASP3, ATF6, ATP5ME, and ELOVL5), during bovine embryo production. These candidates are relevant to pluripotency, cell programming, postcryopreservation viability, embryonic quality, lipid metabolism, and mitochondrial activity.
Materials and Methods
Cumulus-oocyte complexes (COCs) collection, selection and maturation in vitro
Mature Egyptian cow ovaries were collected from Benisuef slaughter house in sterile modified Dulbecco’s phosphate saline (D-PBS; pH=7.2) containing100 μg/mL streptomycin and 100 IU/ mL penicillin at 37°C. They were rinsed in D-PBS then in normal saline. Oocytes were harvested from follicles (3-7 mm) using 18-gauge needle tied to a disposable 10-ml syringe within 3-5 h after slaughtering. Evenly granulated oocytes surrounded with homogeneous cytoplasm and at least two layers of compact cumulus cells were chosen for maturation. COCs were washed in D-PBS, then in in- vitro maturation medium (IVM) that contained TCM-199 (Gibco) supplemented with 10% calf serum (Gibco) and 50 μg/mL gentamycin (G-1272 Sigma). The COCs were cultured in groups of 10-20/100 μL drop of IVM medium in Petri-dishes (Nunclone, Roskilde, Denmark) with mineral oil (M-4810, Sigma) overlay for 22 h at 38.5°C, 5% CO2 and 95% humidity.
In vitro embryo production
Spermatozoa were treated according to Niwa and Ohgoda (1988). Frozen straws were thawed at 35-37°C for 30 sec in a water bath. Sperms were washed in BO medium (Brackett and Oliphant, 1975), containing10 μg/mL heparin and 2.5 mM caffeine without BSA (Sigma, St. Louis, MO) by centrifugation at 800×g for 10 min. To optimize the spermatozoa concentration to 12.5x106 sperm, the sperm pellets were diluted with BO containing 20 mg/mL bovine serum albumin. Oocytes were washed before introducing to the sperm in BO medium with 10 mg/mL BSA, then added to 100 μL droplets of diluted sperm (about 5-10 oocytes/droplet). The oocytes and spermatozoa were co-cultured at 5% CO2, 95% humidity, and 38.5°C for 5 h. The oocytes were then washed several times in TCM-199 to remove the associated spermatozoa. Two-day embryos were then cultured in a medium with Lipid-reducing chemical, phenazineethosulfate (PES). The most appropriate concentrations of PES (0.3 mM) were selected by Barceló-Fimbres and Seidel (2007) for bovine embryo culture that consisting of TCM-199, 50 μg/mL gentamycin and 10% serum. A group of embryos without PES was left as a control. After 72 h of culture, cleavage was determined, and embryos were counted on day 7 (El-Naby et al., 2016).
Vitrification and warming of embryos
In TCM 199 supplemented 20% fetal calf serum, the vitrification solutions were produced. Embryos were vitrified in 1.75 M EG + 1.75 M DMSO for 2.5 min (step one), and then in 3.5 M EG + 3.5 M DMSO for 45 sec (second step) in 0.25 mL straws. Immediately, straws were cooled in liquid nitrogen vapor for 1 min before storing in liquid nitrogen for 30 days. Straws were warmed by holding for 10 sec in air, 30 sec in water at 37°C, and flicked four to six times to mix columns. Then, embryos were washed in 0.5 M galactose for 5 min at room temperature. In the end, the embryos were flushed five times in TCM plus 5% FCS and cultured at 38.5°C, 5% CO2 for further 24 h (Mahmoud et al., 2015).
Survival assay
Embryos were morphologically examined after warming and the viability was evaluated by culturing for 24 h. The embryos developed to advanced stages, were considered to be survived. The morulae that developed into more advanced stage (blastocysts) and the blastocysts that re-expanded were considered as surviving (Mahmoud et al., 2015). After evaluation, embryos were classified into a good or a poor. The embryos (blastocyst and morula) were transferred to a small Eppendorf tube and stored at -80°C for RNA extraction.
In-silico analysis for the identified candidate miRNAs
The differentially expressed genes in bovine embryos were uploaded into miRNA prediction tools such as: DIANA-microT v3.0 (http://diana.cslab.ece.ntua.gr/microT/), miRecords (http://mirecords.biolead.org/), and miRWalk (http://mirwalk.umm.uni-heidelberg.de/). Then the miRNAs hits were filtered based on their probable relevance for pluripotency, cell-programming, post cryopreservation viability, embryonic quality, lipid metabolism, and mitochondrial activity at least in four different search algorithms. Results showed that bta-let-7b, bta-miR-26 and bta-miR-205, as potential targets. Interestingly, bta-let-7b, bta-miR-26 and bta-miR-205 were mentioned by Kropp and Khatib (2015); Tesfaye et al. (2018); Salilew-Wondim et al. (2020), who revealed that these miRNAs were targeting lipid metabolism, as well as mitochondrial activity genes.
RNA isolation, cDNA synthesis (for large and small RNA), and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from control and PES treated embryos; five independent biological replicates (10 pooled blastocysts/each) using miRNeasy mini kit (Qiagen, Hilden, Germany). The isolated total RNA was done according to the manufacturer’s protocol. To exclude any potential genomic DNA contamination, the extracted RNA was treated to on-column DNA digestion with the RNase-free DNase kit (Qiagen, Hilden, Germany), and then stored at 80°C. The total RNA concentration was measured using a Nano Drop 2000/c spectrophotometer (Thermo Fisher Scientific, Wilmington, USA). Furthermore, the integrity of RNA was assessed using 1.5% agarose electrophoresis stained with ethidium bromide. One μg RNA was reverse transcribed into cDNA (for large RNA), using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher scientific, USA), and then the mixture was then run in a thermocycler (Bio-Rad, USA) programmed for 42°C for 60 min, 70°C for 5 min, hold at 4°C. The cDNA was kept at −20°C. The specific primer for each transcript was constructed using Primer3 program version 4.0 (http://primer3.ut.ee/), according to Rozen and Skaletsky (2000), and specifics of primers are shown in Table 1. Quantitative real-time PCR of mRNAs was done in StratageneMx 3000P instrument (Agilent Technologies, USA), using a Maxima SYBR Green/ROX qPCR Master Mix (2×), (Thermo Fisher scientific, USA) and the following conditions: 95°C for 10 min, 40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. At the end of the run, the melting curve was examined to determine the specificity of the amplification. The comparative threshold cycle (ΔΔCt) method was used to evaluate the data and normalization was done using the geometric means of two housekeeping genes GAPDH, β-actin (ACTB). Around 10 ng of total RNA was reverse transcribed via Multi Scribe reverse transcriptase (for small RNA). The RT primers were added separately for each miRNA (let-7b, miR-26, and miR205) according to the supplier’s instructions. Real-time PCR was done in a volume of 10 μl, using 0.7 μl of RT product, 0.5 μl of particular primers with probes (Table 2), and TaqMan Universal PCR Master Mix II (Thermo Fisher Scientific, Wilmington, USA). Amplification was carried out using a StratageneMx 3000P instrument (Agilent Technologies, USA) with initial denaturation for 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C with. The data were examined by the comparative threshold cycle (ΔCt) method and normalization was analyzed using geometric means of 5S, and U6.
Table 1: The primers used for qRT-PCR analysis.
| Gene | Sequence 5´ to 3´ | Accession no | Product size (bp) | Annealing oC |
| CASP3 |
F: ACTGTGGTATTGAGACAGACA R: CGTACTTTTTCAGCATCTCAC |
XM_006075118.1 | 175 | 50 |
| OCT4 |
F: CAGAAGAGGATCACACTAGGAT R: GTCTCTGCCTTG CAT ATCTC |
NM_174580.3 | 212 | 53 |
| ACTB |
F: GGCATTCACGAAACTACCTT R: CAATCCACACGGAGTACTTG |
NM_001290932.1 | 198 | 55 |
| GAPDH |
F: CTACATGGTCTACATGTTCCAG R: CCTTCTCCATGGTAGTGAAGA |
XM_006065800.1 | 200 | 50 |
| DNMT |
F: AGAGTAAGACCAGGAACACAC R: CTAGCTAGATCTTTGGGTTGAC |
XM_015471993.1 | 213 | 55 |
| ATP5ME |
F:CGCCAAGCGCTACAATTA R:GAAGGGCAGGCTCACTTCAATA |
NM_176639.2 | 150 | 54 |
| ELOVL5 |
F:ATGCTCAACATCTGGTGGTTC R:GGATGATGGTCAGCACAAACT |
NM_001046597.1 | 199 | 55 |
| ATF6 |
F:AAGACAAGCCCATCATTGGT R:TGATTGTTTTTGCTGGAACG |
XM_024989877.1 | 162 | 51 |
Table 2: List of miRNAs names, miRbase accession numbers and their mature sequences.
| miR name | Accession number | Mature miRNA sequence | |
| bta-let7-b | MIMAT0004331 | UGAGGUAGUAGGUUGUGUGGUU | |
| bta-miR-205 | MIMAT0003545 | UCCUUCAUUCCACCGGAGUCUG | |
| bta-miR-26 | MIMAT0003516 | UUCAAGUAAUCCAGGAUAGGCU | |
Statistical analysis
Eight replicates in embryo development data and four replicates in vitrified embryos in both treated and control groups were statistically analyzed by ANOVA using statistical software SPSS version 16.0. The LSD test was used to compare the means. At P<0.05 level, differences were considered significant.
The Norm Finder has been used to select the most stable reference gene for mRNA and the miRNAs expression profile (Andersen et al., 2004). All data were tested for homogeneity using Gaussian distribution; the expression of selected genes as well as miRNAs was analyzed using unpaired t test with Welch’s correction (Graph Pad Software, Inc., San Diego, CA, USA). The data is graphed as the mean±standard error of the mean (S.E.M), with P values <0.05 were considered statistically significant. GraphPad Prism 9.0 was employed for performing statistical analysis and generating bar graphs.
Results
Table 3 shows that there was no significant difference in percentage of the cleavage and blastocyst between PES (0.30 μM) and control group. However, the rate of morula was significantly greater in treated (P<0.01) compared to control group.
With respect to the vitrified embryos evaluated directly after warming (Table 4), the percentage of good quality embryos was significantly (P<0.05) higher in treated than control groups, while the rate of dead embryos were significantly (P<0.01) higher in control than treated groups. The percentages of morphologically poor quality embryos did not differ significantly between the two groups.
Morphologically normal vitrified embryos were evaluated also after 24 h of culture (Table 4), the rate of good viable embryos was significantly (P<0.01) greater in treated than control groups. The rate of poor and dead embryos did not differ significantly between the two groups.
Expression profiling of pluripotency, cell-programming, embryo quality associated genes
The OCT4 mRNA was shown an increase (P<0.001) in the treated good embryo compared to control (untreated) groups. Also, OCT4 transcript was up-regulated (P<0.001) in the treated poor embryo compared to control (untreated) groups. The DNMT1 gene increased (P<0.001) in the treated good embryo compared to control (untreated) groups. Also, DNMT1 transcript was up-regulated (P<0.05) in the treated poor embryo compared with control (untreated) groups. The CASP3 mRNA was not shown any significant changes in the treated good embryo, in comparison with control untreated groups. While, there was an increase (P<0.05) in the relative expression of CASP3 gene in treated poor embryo compared to control groups (Figure 1).
Relative abundance of lipid metabolism, mitochondrial activity, and endoplasmic reticulum stress associated genes in bovine embryos
The ATP5ME gene was down-regulated (P<0.05) in the treated good embryo in comparison with control untreated groups. No significant changes were noticed in the relative abundance of ATP5ME transcript between the treated poor embryo and control untreated groups. The ELOVL5 mRNA decreased (P<0.01) in the treated good as well as poor embryos, compared to control untreated good and poor embryos, respectively. The ATF6 transcript was not revealed any significant changes among different groups (Figure 2).
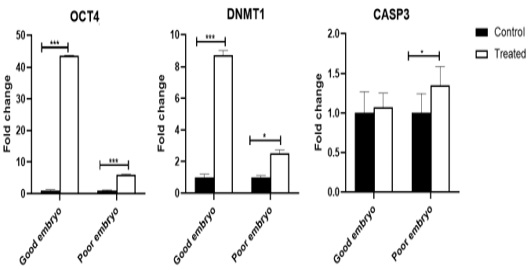
Figure 1: Expression profiling of pluripotency, cell-programming, embryo quality associated genes. Stars represent statistical significance level at*; P< 0.05, ***; P< 0.001.
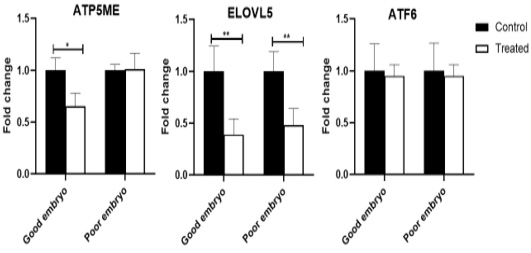
Figure 2: Relative abundance of lipid metabolism, mitochondrial activity, and endoplasmic reticulum stress associated genes in bovine embryos. Stars represent statistical significance level at *; P< 0.05, **; P< 0. 01.
Table 3: Developmental competence of cattle oocytes after culture supplementation with Phenazineethosulphate (Mean ± SE).
| Blastocyst no (%) | Morulae | Cleavage no (%) | Total No. of inseminated oocytes | parameter |
| 198 (18.78±7.4) | 432 (35.7±1.9) | 636 (65.7±2.8) | 936 | Control |
| 204 (23.5±3.3) |
504 (44.8±2.1*) |
882 (70.2±1.9) | 1392 | 0.30 μMPES |
Percent from total inseminated oocytes. No.= number; t –test (*P<0.01, 8 replicates).
Table 4: Cryotolerance of cattle embryos after culture supplementation with Phenazineethosulphate (Mean ± SE).
| Morphologically normal embryos | No. of vitrified-warmed embryos | Parameter | |||||
| 24 h after warming No (%) | Directly after warming No (%) | ||||||
| Dead | Poor | Good | Dead | Poor | Good | ||
| 70 (31.1±5.7) | 35 (19.5±3.6) | 101 (49.3±4.9) |
14 (7.1±0.8**) |
70 (31.0±5.2) | 122 (61.9±5.1) | 206 | Control |
| 52 (19.8±1.6) | 44 (17.5±1.2) |
166 (62.7±1.8*) |
6 (1.5 ±0.9) | 54 (19.9±2.5) |
202 (78.5±2.6*) |
262 | PES (0.30 μM) |
t –test (*P<0.05- **P<0.01, 4 replicates).
The expression pattern of miRNAs (Let-7b, miR-26, and miR-205), which are targeting CASP3, DNMT1, and ELOVL5 in bovine embryos
The Let-7b was over-expressed (P<0.001) in the treated good as well as poor embryos compared to control (untreated) good and poor embryos, respectively. The miR-26 was suppressed in the treated good (P<0.001) as well as poor (P<0.01) embryos compared to control (untreated) good and poor embryos, respectively. The miR-205 increased in the treated good (P<0.001) as well as poor (P<0.01) embryos compared to control (untreated) good and poor embryos, respectively (Figure 3).
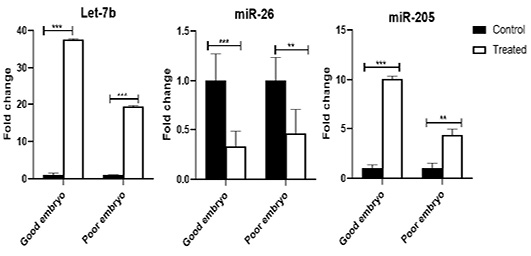
Figure 3: The expression pattern of miRNAs (Let-7b, miR-26, and miR-205), which are targeting CASP3, DNMT1, and ELOVL5 in bovine embryos. Stars represent statistical significance level at **; P< 0.01, ***; P< 0. 001.
Discussion
Lipid droplets, in oocytes and embryos are considered one of the main factors associated with poor cryosurvival. Within the last decade, different trials have been done to reduce oocytes lipid droplets using mechanical and chemical removal by using delipidating agents (Barrera et al., 2018). The current work aimed to investigate the influence of phenazineethosulfate (PES) supplementation in culture media on the selected miRNAs (miR-205, miR-26a-5p, and let-7b) and their target genes (OCT4, DNMT, CASP3, ATF6, ATP5ME, and ELOVL5), during bovine embryo production. Many intrinsic factors are believed to have harmful effects in some mammalian species and from these factors; lipid content of preimplantation embryos. Besides, cytoplasm of developing embryo of cattle and sheep contains high lipids compared to other species (McEvoy et al., 2000). Our data showed that the addition of PES substrate to maturation media significantly enhanced the cleavage rate and viability of cattle embryos. These findings were in agreement with the results showed by many authors (De La Torre-sanchez et al., 2006; Barceló-Fimbres and Seidel, 2007; Sudano et al., 2011; Ghanem et al., 2014) who demonstrated that PES had improved embryo quality and their cryotolerance. In support to this notion, the mitochondrial membrane potential of early blastocysts has increased dramatically (Romek et al., 2011) as the oxidation rate of various fatty acids increased (Tsujii et al., 2001). In our study, morula and blastocyst quality improved in the presence of PES compared with the control counterpart. These findings were in agreement with Sudano et al. (2011) who found that embryos cultured with PES had greater hatching rates. Improvements in embryo quality are due to a reduction in the cytoplasmic lipid content, thus reducing the production of free radicals within the embryo lipid peroxidation (Seidel, 2006; Barceló-Fimbres and Seidel, 2007).
MicroRNAs (miRNAs) are important regulators of cellular differentiation and dedifferentiation. They involve in many developmental processes including; embryo development and preimplantation function by balancing pluripotence with differentiation in the embryo and embryo stem cells so understanding miRNAs regulatory mechanisms during embryogenesis could be used as prognostic molecular markers. In contrast, dysregulation of key miRNAs could alter the genes regulating embryonic development (Reza et al., 2019; Salilew-Wondim et al., 2020). To obtain a good as well as viable embryo, expression of several genes involving transcriptional and post-transcriptional (mRNA turnover, processing, storage and translation) must be regulated (Lasko, 2011; Baranyai et al., 2015). Under in vitro conditions, the changes in miRNAs profile are fully related to the environment of in- vitro maturation and/or materials supplementation (Xiao et al., 2014). Here in the current work, the expression pattern of miRNAs (Let-7b, miR-26, and miR-205), which are targeting CASP3, DNMT1, and ELOVL5 in bovine embryos was affected by addition of PES material compared to control groups. Previously, it was found that transfection of miR- 26b in breast cells showed declined expression of DNMT3B. Furthermore, transfection of miR-26b antagomiR has demonstrated an over-expression of DNMT3B, indicating that this miRNA is involved in DNMT3b regulation (Sandhu et al., 2012). Many miRNA identified as differentially expressed miRNA regulate genes associated with cellular development (Kropp et al., 2014). Some studies have reported the involvement of miRNAs in embryogenesis and renewing of cells (Mondou et al., 2012). Childs et al. (2009) reported that miRNAs expression levels (miR-205 and let-7d) can be used as prognostic markers of cells survival and recurrence. The miR-26a is abundantly expressed during follicular growth and development in cow ovarian tissues (Salilew-Wondim et al., 2014). Furthermore, miR-205 is an important miRNA linked to adipogenesis and lipid metabolism (Cui et al., 2014). It is considered as an important regulator in controlling hepatic lipid metabolism through its negative effect on hepatic mRNA expression (Tao et al., 2018). The level of miR-205 expression is increased in hepatoma cells during adipogenesis, and it reduced the levels of glycogen synthase kinase 3 beta in preadipocyte cells (Yu et al., 2014).
Evaluation of morphology and investigation of genes expression is an essential step during early embryonic development (Lonergan et al., 2003; Cote et al., 2011). In the present study, it was noticed that overexpression of genes related to pluripotency and cell reprogramming in the treated good embryo compared to control (untreated) groups, while the genes related to lipogenesis had been down-regulated. The pattern of DNA methylation is thought to have an important role in gene expression regulation and the expression of imprinted genes, which is important during oocyte maturation and early stages of embryonic development (Lodde et al., 2009). The current findings revealed that addition of lipid metabolism regulator increased DNMT expression. DNMT is necessary for the maintenance of DNA methylation profiles, particularly imprinted gene methylation, throughout the early stages of embryonic reprogramming period to avoid defects in developmental pathway and consequently, onset of adverse health outcomes in offspring (Breton-Larrivee et al., 2019). OCT4 is well known as one of the master regulators of pluripotency and stem cell formation during the early phases of embryonic development (Sadeesh et al., 2016). The present data showed significant over-expression of OCT4 mRNA resulted from addition of PES material. In vitro, OCT4 is necessary for the creation and maintenance of embryonic stem cells as well as the pluripotent reprogramming of somatic cells. However, in vivo, it prevents ectopic trophoblast development in early embryos (Bin et al., 2014). Besides, OCT4 transcript presents in all cells of embryos at the morula stage, it is expressed strictly in inner cell mass (ICM) cells, mediated by the action of gene products expressed on trophectoderm cells (Ralston and Rossant, 2008).
Inhibition of caspase, may prevent cryopreservation induced aberrant apoptosis and increase IVP bovine embryos cryotolerance (Pero et al., 2018). In the present study, the expression profile of CASP3 transcriptin good embryos was not significantly affected in response to lipid modulating. During cryopreservation processes, several variables including mechanical/osmotic injury and oxidative stress change the physical properties of cellular structures, resulting in the activation of the apoptotic pathways and cell death (Paash et al., 2004). A pivotal role of apoptosis in cell cryoinjury has been identified by Stroh et al. (2002), who revealed that cryopreservation promotes the pro-apoptotic activity of caspase 3 in hematopoietic cells. Sudano et al. (2011) found that adding PES to culture media from Day 2.5 to Day 4 decreased lipid content, increased blastocoele re-expansion after vitrification, reduced apoptosis in vitrified blastocysts and improved embryo quality. Our results showed that low expression of genes responsible for lipid formation in response to the addition of lipid scavenger in maturation and culture media. Excessive accumulation of saturated fat is associated with mitochondrial and endoplasmic reticulum damage with induction of oxidative stress (Wu et al., 2011). The evaluation of the expression of genes associated with lipid metabolism has been carried out to predict the ability of embryos to respond to substances, which may generate positive responses to the IVP embryos resistance to the cryopreservation process (Takahashi et al., 2013; Batista et al., 2014; Ghanem et al., 2014; Baldoceda et al., 2015). The ELOVL5 plays an important role in the determination of embryonic lipid droplet content. Blockage of ELOVL5 translation revealed a clear effect on the expression of particular lipids and promoted increased deposition of cytoplasmic lipid droplet (Lanzarini et al., 2021). A previous study concluded that overexpression of ELOVL5 gene at the morula stage preceded an increased abundance of a series of lipids at the blastocyst stage (Sudano et al., 2016). Stress coping mechanisms are essential to reduce or overcome damage caused by environmental changes (Michalak and Gye, 2015). In the current study, there was no change in the expression of gene related to endoplasmic reticulum stress of produced embryos treated with PES. During embryonic development, embryo secretes growth factors that promote embryonic survival during the stage of blastocyst, where cell number increases by the activation of transcription as well as protein synthesis (Abraham et al., 2012). Additionally, ATF6 has a vital role during differentiation and generate a new strategy to form mesodermal tissues (Kroeger et al., 2018). Furthermore, ATF6 is inducible in oocytes and preimplantation embryos, as a normal part of the cellular adaptive mechanism during the developmental stages of preimplantation embryos (Michalak and Gye, 2015). ATF6 loss disrupts the homeostasis of endoplasmic reticulum(ER)and increased ER stress induced damage and cell death (Chiang et al., 2017). ATF6 dysfunction in mammals leads to pathology in many disease models, including obesity associated with ER stress (Jin et al., 2017). Earlier studies indicate that ATF6 plays a vital role in vertebrate embryogenesis and early development (Jang et al., 2012). A clear change in mitochondrial activity of embryos treated with lipid regulators was found and this change was associated to expression of embryo metabolism candidate marker gene (ATP5ME). Takahashi et al. (2013) also reported increasing in the amount of ATP and expression of metabolic-related genes in embryos treated with delipidating agent. Consequently, mechanism of working of PES is to adjust amount of fatty acid by reduction of NADPH to NADP which activates the pathway of pentose phosphate that leads to glucose metabolism and ATP production (De La Torre-sanchez et al., 2006; Barceló-Fimbres and Seidel, 2007; Sudano et al., 2011).
Conclusions and Recommendations
To the best of our knowledge, this is the first study showed the alterations in miRNAs (Let-7b, miR-26, and miR-205) and their targeting transcripts (CASP3, DNMT1, and ELOVL5) in response to PES addition in embryo culture media. Furthermore, PES at the concentrations of 0.30 µM as a supplement in embryo culture medium had dual effects as a metabolic activator and reduced amount of large lipid droplets in cytoplasm to enhance the blastocysts cryotolerance. Further study is required to investigate the precise regulatory role of these candidates miRNA in response to PES addition in embryo culture media.
Novelty Statement
The supplementation of PES in embryo culture medium reduced lipid droplets and enhanced the blastocysts cryotolerance. The effect of PES was investigated at transcription as well as post-transcription levels.
Author’s Contribution
Yasser H.A. Saber and Sally Ibrahim: conceptualization, methodology, statistics analysis and writing and editing the manuscript. Karima Gh. M. Mahmoud: conceptualization and review the manuscript. Seida A.A. and Ragab: data curation and review the manuscript. Wahid M. Ahmed: statistics analysis and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Informed consent
Ethical approval was not applicable as ovaries were collected from slaughtered animals for local meat consumption.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






