Biochemical and In Silico Characterization of a Recombinant, Highly Thermostable α-Glucosidase from Thermococcus radiotolerans DSM-15228
Biochemical and In Silico Characterization of a Recombinant, Highly Thermostable α-Glucosidase from Thermococcus radiotolerans DSM-15228
Hayam Albalawi1,2, Muhammad Shahid Nadeem1*, Hisham N. Altayeb1,
Saima Iftikhar3, Mariam A. Al-Ghamdi1,4,5, Jalaluddin Azam Khan1 and Ahmed Osman1
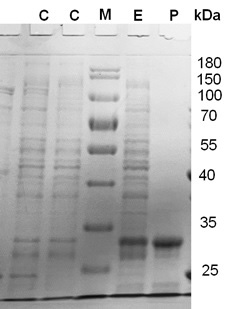
SDS-PAGE showing the expression and purification of α-glucosidase. Lane C, a control experiment (without the gene), Lane-M, Protein marker (Thermofisher - unstained protein ruler). Lane E, experimental with the expression of the gene. P, purified enzyme, the molecular weight of the purified enzyme was found as 30 kDa.
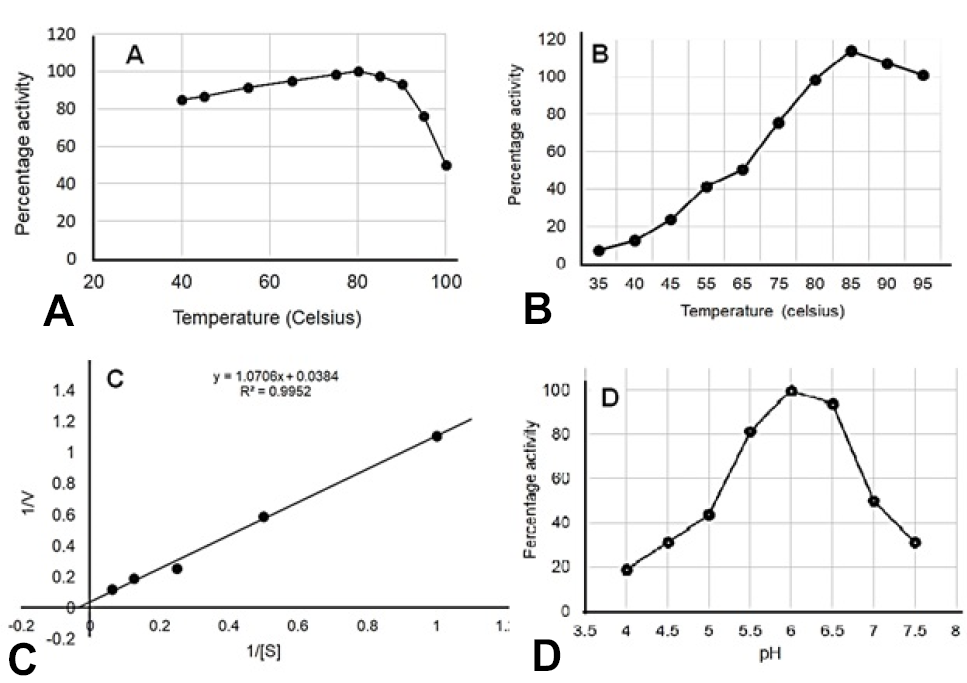
A study of the kinetics of recombinant α-glucosidase. A, Enzyme stability against temperature. B, Effects of temperature on enzyme activity. C, Lineweaver-Burk plot for Km and Vmax. D, Effects of pH on enzyme activity.
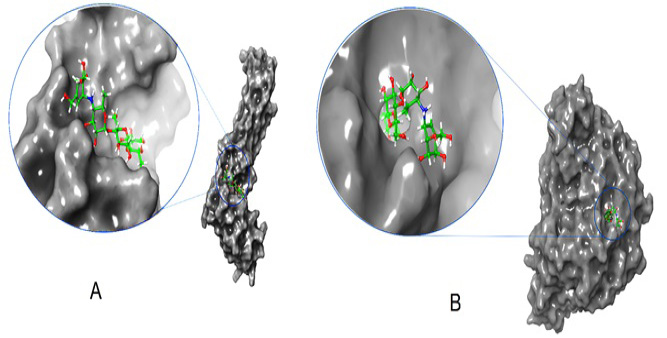
Depicts a 3D interaction of alpha-glucosidase and acarbose. Panel A shows an enlarged model of the complex, highlighting the ligand in the active site of bacterial alpha-glucosidase. Panel B provides a closer view of the complex, with the ligand displayed in the center of human alpha-glucosidase.
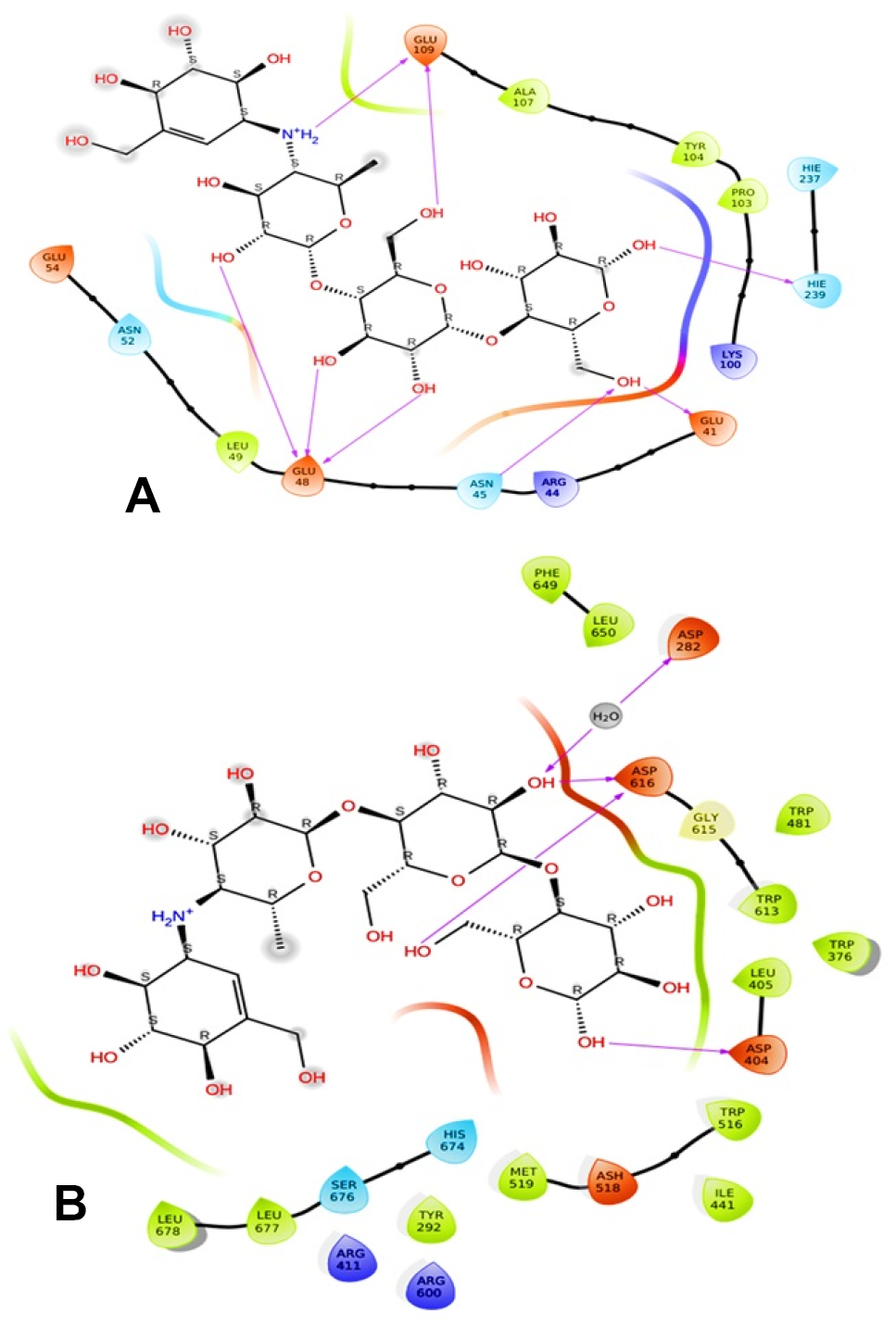
Depicts a 2D interaction of alpha-glucosidase and acarbose. A, enlarged model of the complex, highlighting the ligand in the active site of bacterial alpha-glucosidase. B, provides a closer view of the complex, with the ligand displayed in the center of human alpha-glucosidase.
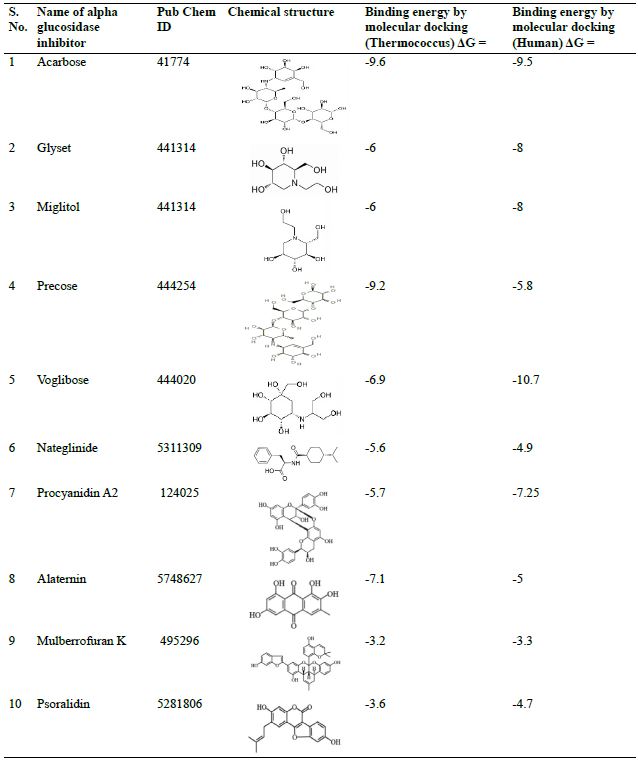
Molecular docking of α-glucosidase with its potential inhibitors a comparison of human and Thermococcus enzyme.