Advances in Animal and Veterinary Sciences
Research Article
Results of Pre-Clinical and Clinical Tests of Organic Hydroxyapatite as Adjuvant of Bacterial Vaccine
Valery Alexandrovich Agoltsov, Olga Mikhailovna Popova, Stepan Yurievich Veselovsky, Alexei Alexandrovich Chastov, Alexander Mefodievich Semivolos, Nataliya Victorovna Solotova*
Department of Veterinary Medicine, Saratov State Agrarian University named after N.I. Vavilov, Russia, 410012, Saratov, Teatralnaya square, Russia.
Abstract | Vaccination continues to be one of the most effective ways to protect against infectious diseases; therefore, there is a need to develop new vaccines that have low cost and are used either once or for long periods of time.The current study aims to test organic hydroxyapatite in bacterial vaccines on laboratory and farm animals and determine its adjuvant and immunogenic properties. In pre-clinical tests, white mongrel mice weighing 18-20g and rats weighing 250-300g were used. Adjuvant (organic hydroxyapatite) separately and as part of vaccines was administered subcutaneously, intraperitoneally and intramuscularly at a dose of 0.5 ml. Clinical tests of adjuvant were carried out on Holstein-Friesian cows and calves. Experimental vaccines were used: 1) a split-conjugated vaccine against animal brucellosis (as antigens it included the components of brucella (Br. Abortus); 2) a vaccine against mastitis of cows. Results showed that PhA, neutrophils and monocytes in the blood smears of the control group were 1.4 times lower, and twice as much compared to the vaccine without the adjuvant. The maximum PhI(FI) was observed in animals immunized with a split-conjugated vaccine against brucellosis of animals with the adjuvant. The disappearance of vaccine antibody titer in the blood after 30 days was stated, which is positive in the diagnosis of brucellosis. The anti-mastitis vaccine with the adjuvant demonstrates prophylactic properties. In conclusion: Organic hydroxyapatite, obtained from the bones of animals by pyrolysis, displays adjuvant and immunogenic properties when used in bacterial vaccines.
Keywords | Adjuvant, Bacterial vaccines, Brucellosis, Mastitis, Organic hydroxyapatite.
Received | December 05, 2018; Accepted | February 27, 2019; Published | May 28, 2019
*Correspondence | Nataliya Solotova, Department of Veterinary Medicine, Saratov State Agrarian University named after N.I. Vavilov, Russia, 410012, Saratov, Teatralnaya square, Russia; Email: [email protected]
Citation | Agoltsov VA, Popova OM, Veselovsky SY, Chastov AA, Semivolos AM, Solotova NV (2019). Results of pre-clinical and clinical tests of organic hydroxyapatite as adjuvant of bacterial vaccine. Adv. Anim. Vet. Sci. 7(7): 583-592.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.583.592
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Agoltsov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Vaccination continues to be one of the most effective and safe ways to protect against infectious diseases (Clapp et al., 2011; Clements and Griffiths, 2002). However, the high price of vaccines and their repeated administration throughout the year are serious deterrents to their use in large numbers of animals. Therefore, there is a need to develop new vaccines that have low cost and are used either once or for long periods of time (1-2 years) in order to facilitate the work of both veterinary workers and cattle breeders (Zuhair BI, 2017) Vaccines enhanced with an adjuvant cause a longer and more intense immune response (Harandi et al., 2009; Peek et al., 2008).
The word “adjuvant” comes from the Latin adjuvara, that is, “to help” (He et al., 2000). The adjuvant capacity of minerals was first discovered by Gleny et al. in 1926, after the precipitation of aluminum sulfate suspension of diphtheria toxoid, which increased the immunogenicity of this drug. Later, a large number of inorganic salts were tested, but alum, phosphate, aluminum hydroxide, aluminum hydroxide hydrophosphate and calcium phosphate were only approved. Adjuvants are substances that, in combination with an antigen, potentiate an immune response (Ablova et al., 2013). The inclusion of adjuvants is one of the best strategies for increasing the effectiveness of the vaccine.
Modern adjuvant technologies tend to focus either on immune stimulating molecules (saponins-QS21, CpG-oligonucleotides, lipopolysaccharides, monophosphorylpyrides A, cytokines), lipid structures (liposomes, virosomes, limits) or (virus-like polymeric particles) or on only conventional adjuvants based on aluminum salts (De Grigorioet al., 2008; Jiang et al., 2004). The adjuvant properties of inorganic salts strongly depend on the nuances of their production processes, which directly affect the depot of vaccine antigens (Contorni et al., 2009).
Many adjuvant vaccines form a depot at the injection site from which the antigen is slowly released, thereby maintaining the antigenic effect of the immune system for a longer time and, as a consequence, provoking a stronger response. For a long time, the prevailing view was that this was the only or the main mechanism of action of immunological adjuvants (Marrack et al., 2009). However, it is now known that vaccine adjuvants act in a variety of different and non-mutually exclusive mechanisms, including stimulation of inflammation and release of cytokines, more efficient delivery of the antigen to the antigen presenting cells (due to their particles, structure and size of less than 10 μm), stimulation of immunocompetent cells by activation of complement.
The average particle size, morphology and surface charge have the greatest effect on the adsorption of antigens to inorganic adjuvants. Adjuvant with an isoelectric point above the physiological pH becomes positively charged at this pH and will readily adsorb negatively charged antigens. Conversely, those with isoelectric points below physiological pH will adsorb positively charged antigens at this pH, so Van der Waals forces and hydrophobic interactions will predominate whenever the isoelectric points of the antigen and adjuvant are similar. Quantitatively, the adsorption will depend on the chemical composition and antigen concentration, the presence of salts or ions, such as those obtained with commonly used buffers, and the pH of the solution obtained (Jiang et al., 2004).
Moreover, the adsorption process can cause structural changes in the antigen that can increase its susceptibility to many host proteases involved in generating immune responses in favor of its presentation by the professional antigen presenting cells (Jones et al., 2005).
Aluminum salts are the most popular and ubiquitous class of vaccine adjuvant. Indeed, potassium sulfate (K(SO4) 2 × 12 H2 O), whose characteristics are very similar to those of aluminum phosphate, were used to prepare tetanus aluminates and diphtheria toxoid to vaccinate people (Lindblad, 2004). Aluminum hydroxide and phosphate, as well as aluminum hydroxyphosphate sulfate, are the only aluminum-based adjuvants that are currently approved for the production of vaccines licensed for clinical use.
Initially, calcium phosphate was considered as an alternative to aluminum-based adjuvants. Subsequently, it was used as an adjuvant in vaccines against diphtheria, tetanus, pertussis, poliomyelitis, tuberculosis, yellow fever, measles and hepatitis B (Gupta, 1998).
Although the properties of calcium salts are similar to those of aluminum, the former has a number of potential advantages. Calcium is a normal component of the human body and animals and is therefore well tolerated. Its ability to adsorb antigens is excellent, and the release of the antigen proceeds only slowly. Finally, it stimulates the induction of IgG, but not IgE antibodies, reducing the likelihood of long-term side effects. However, there have been episodic cases of neurological reactions following the administration of Bordetella pertussis vaccines adsorbed by this adjuvant, therefore the World Health Organization, together with the European Pharmacopeia, recommended an upper safety limit of 1.3 mg calcium / dose.
The empirical formula of calcium phosphate, used in adjuvants, is approximated by Ca3 (PO4)2. X-ray diffraction, infrared spectroscopy, and thermal analysis, among other methods, have shown that calcium phosphate is a non-stoichiometric hydroxyapatite: Ca10x(HPO4) x (PO4)6 x (OH)2 - X (where x is in the range from 0 to 2). The surface charge depends on the pH, and its isoelectric point is 5.5. Therefore, calcium phosphate is negatively charged at physiological pH and adsorbs positively charged antigens through electrostatic interaction. This adjuvant can also adsorb phosphorylated antigens by exchanging ligands through surface hydroxyls.
The adjuvant properties of calcium phosphate strongly depend on the deposition conditions, which are very similar to that described above for aluminum salts. Sediment obtained by rapid mixing of the two reagents adsorbs 100% diphtheria toxoid, but those that are obtained by slow addition only absorb 58% of the same dose. The phenomenon caused by the molar ratios of Ca/P, which can vary from 1.35 to 1.83 depending on the mixing rate.
The synthesized nano-particles of calcium phosphate show better physico-chemical characteristics than traditional drugs (Singh and O’Hagan, 2002). Nano particles give fewer inflammatory reactions at the injection site, higher IgG titers and lower IgE titers compared to aluminum-based adjuvants. These particles are considered a good alternative for immunization schemes involving viral antigens and are already being used in studies with intranasal introduction (Abd el-Rasek et al., 2011). Thus, nano structured calcium phosphate is a good example of how to develop more advanced adjuvants by simply changing the size and morphology of existing compounds.
Since many inactivated vaccines do not have long-term humoral immunity in the animals, it therefore becomes necessary to prolong the immunity for the administration of vaccines with the help of various adjuvants, the most promising among them being organic hydroxyapatite (Ablova et al., 2014; Dubyanskii, 2013; Peek et al., 2008).
So, the goal of research was to test organic hydroxyapatite in bacterial vaccines on laboratory and agricultural animals and determine its adjuvant and immunogenic properties.
Materials and Methods
In pre-clinical trials, white mongrel mice weighing 18-20 g and rats weighing 250-300 g were used. Five groups of mice and rats were formed, 5 animals per group: 1) adjuvant; 2) anti-brucellosis vaccine; 3) anti-mastitis vaccine; 4) anti-brucellosis vaccine + adjuvant; 5) anti-mastitis vaccine + adjuvant. Adjuvant (organic hydroxyapatite) was introduced subcutaneously, intraperitoneally and intramuscularly at a dose of 0.5 ml. separately and as part of vaccines.
Clinical trials of adjuvant (both separately and as part of anti-brucellosis and anti-mastitis vaccines) were carried out on Holstein-Friesian cows and calves. Six groups of cows were formed for 10 animals in each group. Groups 1 and 2 (milking cows) together with groups 3 and 4 (non-milking cows) were injected with anti-mastitis vaccine + adjuvant and pure anti-mastitis vaccine; groups 5 and 6 were control groups. Experimental vaccines were used: 1) a split-conjugated vaccine against animal brucellosis (Compés et al., 2017) (as antigens it included the components of brucella (Br. Abortus); 2) vaccine against mastitis of cows (as antigens it consisted of streptococci (S. agalactiae, S. pyogenes), staphylococci (St. aureus, St. epidermidis) and Escherichia (E. coli). Both vaccines were formaldehyde inactivated.
Observations of mice and rats injected with drugs lasted for 14 days, followed by decapitation. During the first 24 hours, local reactions were observed in the body of mice and rats, for their behavior, motor activity, intake of feed and drinking water. Observations of cows injected with drugs lasted for 180 days. In the same period, studies were conducted on mastitis. From calves, blood was taken at various intervals after immunization with a split-conjugated vaccine against animal brucellosis (with and without adjuvant), serological studies were carried out (agglutination test, complement fixation test, competitive enzyme-linked immunosorbent assay) and hematological analysis with phagocytosis study was made (Avila-Calderón et al., 2013; Khwaja et al, 2013; Erko et al., 2014; Bayemil et al., 2015; Bamaiyi, 2016; Chayu and Paride, 2017).
When taking blood and carrying out vaccination, the animals were fixed in a classing race. The vaccine was injected into the middle third of the neck, carefully holding the animal’s head (Meka-Mechenko et al., 2013; Lyamkin et al, 2013; Francesca et al., 2016; Praud et al., 2016). The injection site was treated with a cotton pad dipped in 70% alcohol. Blood was taken from the sub caudal vein, using sterile needles, to which vacuum tubes were attached. The place of blood collection was also sterile treated with a cotton pad dipped in 70% alcohol (Amano et al., 2010). Blood was delivered to the Engels Veterinary Laboratory during 3 hours from the time of collection, where brucellosis studies were carried out by agglutination and complement fixation tests (Marie, 2016).
The preparation of the adjuvant (hydroxyapatite) was carried out by rapid pyrolysis from the hip bones of cattle. A detailed method of preparation is described in Patent Application No. 2017110631 of the Russian Federation of March 29, 2017. Adjuvant was obtained from WestIntech Ltd., Saratov, Russia.
PhA (FA) of neutrophils and monocytes was determined according to the formula: FA = 100 * F / 100, where F is the number of neutrophils and monocytes of white blood cells participating in phagocytosis (which captured a certain number of microbes).
The PhI(FI) was determined by the average number of phagocytosed microbes per one active leukocyte. This was done to show the intensity of phagocytosis. To determine the FI, the same blood smears were used, according to which the FA of leukocytes was determined. In specimen prepared by the method described above, 100 leukocytes and the number of microbial bodies they absorbed were counted. FI was calculated by dividing the number of phagocytosed bacteria by the number of active leukocytes.
The vaccine against mastitis in cattle was tested on cows. Preliminary obstetric and gynecological prophylactic medical examination of the productive population of the dairy complex was conducted. Based on the results of the clinical examination, a group of 60 clinically healthy cows was selected. The group included 30 milking cows and 30 non-milking cows. A group of 60 animals was divided into 4 experimental and 2 control groups. Each group had 10 cows.
Cows of experimental groups were injected with the vaccine against mastitis of cattle, both with adjuvant and without adjuvant in a dose of 5.0 ml. intramuscularly. The injection site was sheared and treated with 70% alcohol. After introduction of the drug, the animals were observed for 2 hours. Daily (morning and evening) for 10 days their body temperature, pulse, respiratory rate and scar reduction were measured. There were no abnormalities.
Experimental and control animals were followed up for 9 months, starting from the moment of drug introduction. Twice a month during the whole observation period, the animals were examined for the presence of a pathological process in the mammary gland and milk samples were taken for research.
Hidden mastitis was diagnosed with a 5% solution of dimastine, 10% of the solution of mastidine and milky control plates.
Experimental research, maintenance, care and euthanasia were carried out according to the requirements of the “European Convention for the Protection of Vertebrates used for experiments and other scientific purposes” (1986).
To carry out the in vivo experiment, dry powders with a mass of 0.75 grams obtained by rotary and rotational vortex grinding were used which were a sinter of micro- and nano particles with a fraction of 0.2-3 μm and a maximum in the distribution curve was below 1 μm (Figure 1).
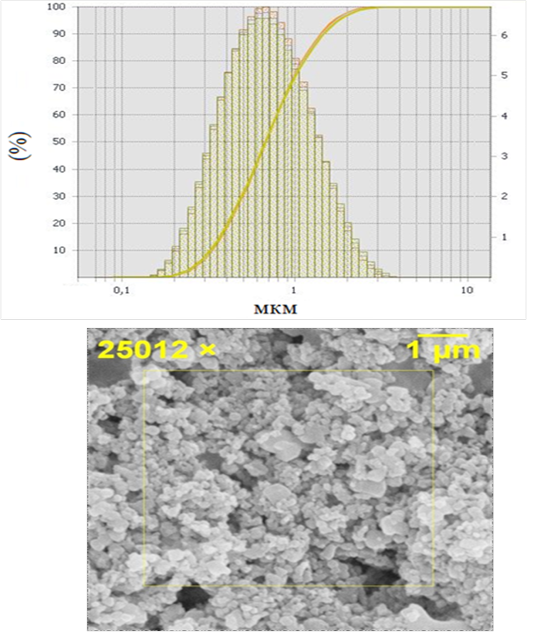
Figure 1: Hydroxyapatite powders 0.2 - 3 μm, where a) granulometric analysis, b) electron microscopy
The powders were further fractionated by sedimentation from an aqueous suspension. The separated non-sedimented fine part was used for further experiment in the form of a colloidal suspension (composition 0.01 g of powder, 1 ml of physiological solution) and processed by ultrasound (US). The ultrasonic treatment provided additional destruction of the agglomerated particles.
The powders were sterilized at a temperature of 200 ° C for 30 minutes. Dry powders with a mass of 0.75 grams were dissolved in 5.0 ml of the vaccine (saline).
The split-conjugated vaccine against animal brucellosis initially contained aluminum hydroxide, which we replaced with hydroxyapatite, in order to enhance the humoral immune response by the body of animals (Marie, 2016). Aluminum hydroxide is known to be widely used as adjuvant for many vaccines, but is relatively toxic to animals (Mikhalishin and Mamkov, 2008).
To verify the data obtained Student t-test was used, and the statistical significance level of cow parameters was (p≤0.05).
Results
After the introduction of the adjuvant in mice and rats, local and general negative reactions were absent. Histological studies of the tissues of the injection site and the tissues of the internal organs did not detect any changes.
Studies of phagocytosis indices by neutrophils and blood macrophages of calves immunized with a split-conjugated vaccine against animal brucellosis, with adjuvant and without it are presented in Table 1.
FA using a split-conjugated vaccine against animal brucellosis (without adjuvant) was: FA = 100 * 25/100 = 25%;
FA neutrophils and monocytes when using a split-conjugated vaccine against brucellosis of animals (with adjuvant) was: FA = 100 * 51/100 = 51%;
FA neutrophils and monocytes without vaccine (control) was: FA = 100 * 31/100 = 31%.
From the results obtained, it follows that neutrophils and monocytes in blood smears obtained from animals after the application of a split-conjugated adjuvant vaccine have the best PhA (51%). FA of neutrophils and monocytes in the blood smears of the control group was 1.4 times lower, and compared to the vaccine without adjuvant 2 times lower.
FI = 39/25 = 1.56 - PhIof neutrophils and monocytes when using a split-conjugated vaccine against brucellosis of animals without adjuvant; FA = 108/51 =
Table 1: Results of phagocytosis studies with neutrophils and blood monocytes, after vaccination with an anti-brucellosis vaccine
|
№ test |
Experiment (vaccine) | Control (non-vaccinated) | ||||
| without adjuvant | with adjuvant | |||||
| Number of phagocytes | Number of microbes phagocytosed | Number of phagocytes | Number of microbes phagocytosed | Number of phagocytes | Number of microbes phagocytosed | |
| 1. | 16 | 20 | 44 | 120 | 24 | 44 |
| 2. | 40 | 72 | 52 | 96 | 36 | 40 |
| 3. | 28 | 64 | 52 | 76 | 30 | 40 |
| 4. | 16 | 16 | 49 | 100 | 28 | 42 |
| 5. | 32 | 40 | 53 | 116 | 36 | 46 |
| M±m | 25±4 | 39±5 | 51±3 | 108±8 | 31±4 |
43±2 |
(p≤0.05)
Table 2: Results of a general analysis of calves’ blood, after introduction of the vaccine with adjuvant
| Indicator | Norm | Animal Identification number | ||||
| 2089 | 17002 | 2086 | 2071 | 2090 | ||
|
erythrocytes, х1012/l |
5 – 7,5 | 7,45 | 9,38 | 8,3 | 7,25 | 6,5 |
| hemoglobin, g/l | 90 - 120 | 89 | 91 | 81 | 80 | 81 |
| hematocrit, % | 35 - 45 | 33,4 | 36,97 | 30,52 | 30,31 | 30,41 |
|
thrombocytes, х109/l |
260 - 700 | 204 | 406 | 148 | 50 | 137 |
|
leucocytes, х109/l |
4,5 - 12 | 9,26 | 10,7 | 11,2 | 12,8 | 8,2 |
| lymphocytes | 40 - 75 | (3,7 - 6,9) 6,1 |
(4,2 – 8,0) 6,6 |
(4,5 – 8,4) 7,7 |
(5,2 – 9,6) 6,2 |
(3,3 – 6,2) 5,24 |
| monocytes | 2 - 7 |
(0,2 – 0,6) 0,2 |
(0,2 – 0,7) 1,0 |
(0,2 – 0,8) 1,15 |
(0,3 – 0,9) 0,35 |
(0,2 – 0,6) 0,12 |
| neutrophils | 22 - 39 |
(2 – 3,6) 2,32 |
(2,4 – 4,2) 2,87 |
(2,5 – 4,4) 1,6 |
(2,8 – 5,0) 5,0 |
(1,8 – 3,2) 2,32 |
| eosinophils | 3 - 10 |
(0,3 – 0,9) 0,62 |
(03 – 1,0) 2,5 |
(0,3 – 1,1) 0,82 |
(0,4 – 1,3) 1,24 |
(0,2 – 0,8) 0,47 |
| basophils | 0 - 2 |
(0 – 0,2) 0,01 |
(0 – 0,05) 0,01 |
(0 – 0,2) 0,01 |
(0 – 0,3) 0,01 |
(0 - 0,2) 0,01 |
(p≤0.05)
Table 3: Results of a common blood test of calves immunized with an anti-brucellosis vaccine without adjuvant
| Indicator | Norm | Animal Identification number | ||||
| 2105 | 2085 | 2074 | 2084 | 2091 | ||
|
erythrocytes, х1012/l |
5 – 7,5 | 7,02 | 8,6 | 7,46 | 7,48 | 7,31 |
| hemoglobin, g/l | 90 - 120 | 74 | 89 | 111 | 89 | 83 |
| hematocrit, % | 35 - 45 | 28,97 | 35,0 | 36,4 | 31,23 | 30,6 |
|
thrombocytes, х109/l |
260 - 700 | 559 | 128 | 117 | 115 | 353 |
|
leucocytes, х109/l |
4,5 - 12 | 10,6 | 10,9 | 10,4 | 9,7 | 9,2 |
| lymphocytes | 40 - 75 |
(4,2 – 8,0) 6,06 |
(4,3 – 8,0) 6,13 |
(4,2 – 7,9) 8,75 |
(3,9 – 7,3) 7,35 |
(3,7 - 6,9) 6,31 |
| monocytes | 2 - 7 |
(0,2 – 0,8) 0,08 |
(0,2 – 0,8) 0,29 |
(0,2 – 0,7) 1,34 |
(0,2 – 0,7) 0,09 |
(0,2 – 0,6) 0,89 |
| neutrophils | 22 - 39 | (2,3 – 4,1) 3,73 |
(2,4 – 4,3) 3,9 |
(2,3 – 4,0) 0,25 |
(2,1 – 3,8) 1,41 |
(2 – 3,6) 1,43 |
| eosinophils | 3 - 10 |
(0,3 – 1,0) 0,69 |
(0,3 – 1,0) 0,59 |
(0,3 – 1,0) 0,07 |
(0,3 – 1,0) 0,85 |
(0,3 – 0,9) 0,53 |
| basophils | 0 - 2 |
(0 – 0,2) 0,04 |
(0 – 0,2) 0,01 |
(0 – 0,2) 0,0 |
(0 – 0,2) 0,01 |
(0 – 0,2) 0,02 |
(p≤0.05)
2.12 - PhIof neutro phils and monocytes when using a split-conjugated vaccine against brucellosis of animals with adjuvant;
FA = 43/31 = 1.38 - PhIof neutrophils and monocytes in animals without vaccines (control).
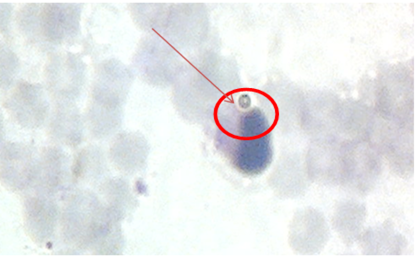
The largest FI was observed in animals grafted with a split-conjugated vaccine against brucellosis of animals with adjuvant, which in our opinion characterizes the activation of the cellular immune response in immunized calves. Activation of the cellular immunity is an important factor of protecting animals from infection by the causative agent of brucellosis. In blood smears, both control animals and animals grafted with a split-conjugated vaccine against animal brucellosis without the use of FI adjuvant are much lower (1.56 and 1.38, respectively), which indicates a lower level of cellular immunity in such animals. The implementation of phagocytosis is shown in Figure 2.
Before the study of phagocytosis, a month after vaccination, a morphological study of blood samples was carried out (general analysis). The results of a general analysis of calves’ blood are presented in Table 2.
The split-conjugated vaccine against animal brucellosis initially contained aluminum hydroxide, which we replaced with hydroxyapatite, in order to enhance the humoral immune response by the body of animals (Mikhalishin and Mamkov, 2008). Aluminum hydroxide is known to be widely used as adjuvant for many vaccines, but is relatively toxic to animals (Nikiforova and Mironov, 2011).
An increase in the number of leukocytes (within the limits of the physiological norm) is established, which is associated with leukocytosis in response to the introduction of the vaccine. There was no clearly defined increase or decrease in granulocytes and agranulocytes. A little lower than the physiological norm is the number of platelets. The number of red blood cells was in the higher range of the physiological norm, and in some samples slightly above normal, which is possible due to the physical stress of animals prior to blood sampling (transhumance). In this case, the amount of hemoglobin in the blood is somewhat reduced, but in the lower limits of the physiological norm.
General blood analysis results of calves immunized with an anti-brucellosis vaccine without adjuvant are presented in Table 3.
In many respects, the morphological pattern of blood in animals after vaccination with a vaccine without adjuvant was similar to the data in Table 2. The number of leukocytes averaged 10,2х109/ l, which was somewhat lower than the number of leukocytes immunized with the vaccine with an adjuvant where the number of leukocytes was 10,4 х109/ l.
The time dynamics, the results of serological studies of blood samples of cattle immunized with a split-conjugated vaccine against animal brucellosis, with adjuvant and without it were analyzed.
The blood for serological reactions was examined after 14, 30 and 75 days after vaccination. The results of serological tests are presented in Table 4.
Table 4: Results of serological studies of calf blood samples, 14 days after vaccination
|
№ |
Animal Identification number | Test Results | |
| AR (agglutination reaction) | CFR (complement-fixation reaction) | ||
| 1. | 2074 | 200 IU | 1/80 |
| 2. | 583 | 200 IU | 1/40 |
| 3. | 2091 | 400 IU | 1/40 |
| 4. | 2086 (A) | 50 IU | 1/20 |
| 5. | 2071 (A) | 200 IU | 1/10 |
|
6. |
2106 | 400 IU | 1/20 |
| 7. | 2090 (A) | 50 IU | negative |
| 8. | 17002 (A) | 100 IU | 1/5 |
| 9. | 2105 | 200 IU | 1/40 |
| 10. | 2084 | 200 IU | 1/40 |
| 11. | 2095 | 400 IU | 1/40 |
| 12. | 2085 | 400 IU | 1/40 |
| 13. | 2089 (A) | 400 IU | 1/80 |
Note: “A” indicates blood samples from calves immunized with vaccine with adjuvant.
After the first blood collection, the presence of agglutinin dilutions was found: 1/100; 1/200; 1/400 and complement-binding antibodies in a 1/5 titer; 1/10, 1/20, 1/40, 1/80, both in blood samples of animals immunized with vaccine without the use of adjuvant and with adjuvant. The data obtained indicate that the vaccine, both with adjuvant and without it, activates humoral immunity by the formation of specific complement-binding and agglutinating antibodies.
The results of repeated serological studies are presented in Table 5.
Table 5: Results of serological studies of calf blood samples, 30 days after vaccination
|
№ |
Animal Identification number | Test Results | |
| AR | CFR | ||
| 1. | 2106 |
1/50 IU (controversial) |
negative |
| 2. | 2085 | 1/200 IU | 1/5 |
| 3. | 2074 |
1/50 IU (controversial) |
negative |
| 4. | 2089 (A) | 1/100 IU | negative |
| 5. |
2084 |
unsuitable | unsuitable |
| 6. | 17002 (A) | 1/100 IU | negative |
| 7. | 2095 | 1/100 IU | negative |
| 8. | 583 | 1/100 IU | negative |
| 9. | 2086 (A) |
1/50 IU (controversial) |
negative |
| 10. | 2091 | 1/100 IU | negative |
| 11. | 2105 |
1/50 IU (controversial) |
negative |
| 12. | 2071 (A) | 1/100 IU | negative |
| 13. | 2090 (A) |
1/50 IU (controversial) |
negative |
One month after vaccination, blood serological tests revealed that in almost all blood samples there were no complement-binding antibodies (AB) in the CFR. Agglutinins were present in the overwhelming majority of cases in a titer 1/100, sometimes 1/50 (controversial reaction). Thus, the animals react negatively in AR and CFR. Doubtful results (1/5) were found only in one reaction of CFR. All this indicates that the complement-binding AB in the blood can only be found for short time (disappear from the blood after 30 days).
The results of repeated serological studies are presented in Table 6.
When the blood was examined 2.5 months after the vaccination, it was found that both in AR and in CFR negative results were obtained for brucellosis. Only one sample when using CFR is doubtful (1:50), all the others are negative.
Table 6: Results of serological studies of calf blood samples, 75 days after vaccination
|
№ |
Animal Identification number | Test Results | |
| AR | CFR | ||
| 1. | 583 | negative | negative |
| 2. | 2089 (A) | negative |
negative |
| 3. | 2095 | negative | negative |
| 4. | 2106 | negative | negative |
| 5. | 2090 (A) | negative | negative |
| 6. |
2105 |
negative | negative |
| 7. | 2086 (A) | negative | negative |
| 8. | 2071 (A) | negative | negative |
| 9. | 2084 | negative | negative |
| 10. | 2091 | negative | negative |
| 12. | 2085 | 1/50 (controversial) | negative |
| 13. | 2074 | negative | negative |
| 14. | 17002 (A) | negative |
negative |
Note: “A” indicates blood samples from calves immunized with vaccine with adjuvant.
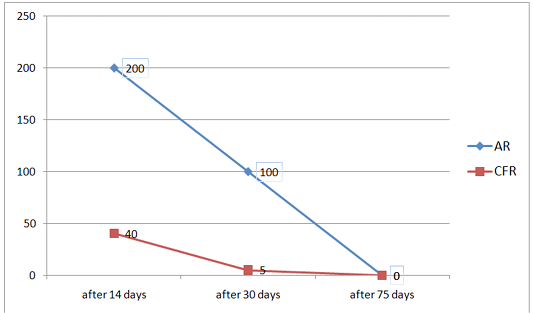
Figure 3: Change in antibody titer in AR and CFR during a three-time study of blood samples from calves after vaccination
In the previous experiment, we tested the same vaccine, but aluminum hydroxide was used as an adjuvant. After 2.5 months in the blood of animals, antibodies were absent, as in the current experiment, which indicates that these two adjuvants have the same effect on the humoral immune response in animals. In addition to the above-mentioned adjuvants, oil-based adjuvants are used in veterinary practice, for example, from Seppic (France). These adjuvants are often reactogenic, since swelling is observed at the injection site, which does not disappear for a long time, as a result of which animals suffer (Veselovsky et al., 2016).
Based on the data shown in Tables 4 to 6, a graph was constructed that characterizes the dynamics of changes in
Table 7: Results of preventive efficacy of the vaccine against bovine mastitis
| Groups of Cows | Number of experimental/control animals | Experiment (vaccinated) | Control | ||
| Infected with Mastitis after vaccination with adjuvant | Infected with Mastitis after vaccination without adjuvant |
Infected with Mastitis
|
|
||
| Milking | 20/10 | 0 | 1 | 3 | |
| Non-milking | 20/10 | 0 | 1 | 3 | |
antibody titer for 75 days, after the introduction of the vaccine, with an adjuvant in AR and CFR (Figure 3).
The results of preventive efficacy of the vaccine against mastitis in cattle are presented in Table 7.
In the control group, consisting of 10 milking and 10 non-milking cows, 3 milking and 3 non-milking cows fell ill during the observation period.
Discussion
Split-conjugated vaccine against animal brucellosis is a new vaccine that has not yet been studied, and we have not found any articles or other literature on this very vaccine.
Split-conjugated vaccine against animal brucellosis activates a specific humoral immune response by forming complement-binding and agglutinating antibodies, both using aluminum hydroxide adjuvant and using aluminum alum. Antibodies (AR 1: 200, 1: 400, CFR 1/40, 1/80) in high titer are formed 14 days after the administration of the vaccine, but they are not detected 1 month after vaccination at CFR, and 2.5 months later in AR, which does not indicate a prolonged humoral immune response when using both adjuvants. Our data demonstrate that the adjuvants we have tested do not have a strong effect on the strengthening of the humoral immune response of animals. These data differ from the opinion of S. Rybalko, M. Khristova, A. Shapiro, who believe that adjuvants can enhance the humoral immune response (Rybalko et al., 2003).
Some scientists (Bobylev, 2001; Savitsky and Bronnikov, 2015) believe that in order to improve preventive measures against brucellosis, it is necessary to use different types of chemical vaccines at the optimal time for immunization. Efficacy monitoring should be carried out 15 days after vaccine administration. Our opinion is different. We believe that using only one split-conjugated vaccine with adjuvants against brucellosis provides the best results.
General blood analysis after using hydroxyapatite and aluminum hydroxide with vaccine showed a slight increase in the number of leukocytes (within the physiological norm). When using both adjuvants, local and general reactogenicity and allergenicity of animals to the introduced adjuvant were absent. We believe that this is a positive result, because for example, oil adjuvants after use form a swelling at the injection site of the vaccine, which disappears within 5-6 months, which is a negative result, and such adjuvants cannot be used for mass vaccination of animals.
Conclusion
Organic hydroxyapatite derived from animal bones by pyrolysis, subjected to rotational vortex grinding to micro- and nano particles with a fraction of 0.2-3 μm, with additional fractionation by sedimentation from an aqueous suspension and not sedimented with a finely dispersed part in the form of a colloidal suspension and sonicated, with a thermal exposure of 200 ° C for 30 minutes, has adjuvant and immunogenic properties when used in bacterial vaccines. Organic hydroxyapatite does not adversely affect the body of laboratory and farm animals.
Acknowledgments
The authors are thankful to the Rector of Saratov State Agrarian University and to the WestIntech Ltd., Saratov, Russiafor supporting with all essential requirements for present research.
Conflict of Interest
The authors have not declared any conflict of interests.
Authors contribution
Valery Alexandrovich Agoltsov, Olga Mikhailovna Popova designed the experiment. Stepan Yurievich Veselovsky andAlexander Mefodievich Semivolos conducted the statistical analysis, as well as conducting the experimental measurement, Alexei Alexandrovich Chastov and Nataliya Victorovna Solotova contributed by writing the article and reviewing it thoroughly before submission.
References