Advances in Animal and Veterinary Sciences
Research Article
Study the Histological Changes in Intestine of Infected Rabbits with Entamoeba histolytica after Immunization with Klebsiella pneumonia K-Antigen
Karama Tahreer Ahmed Al-Taee*, Khadiji Khleaf Al-Dulaimi
Lecturer Department of Basic Sciences, College of Dentistry, University of Anbar.
Abstract | The purpose of this study is to examine the immunological influence of K-Antigen isolated from (Klebsiella pneumonia), in the intestine of rabbits that were inoculated in the laboratory with Entamoeba histolytica. In our study three groups of rabbits were used. The first group (Group I) has ten animals which were injected, subcutaneous and Intramuscular, with 1 ml of concentrations (0, 10, 20, 40.100) mg/ ml of K-antigen. After two weeks, Group I divided in two Group Ia and Group Ib, Group Ia(five rabbits) were dissected; the histological changes in the intestine were examined. The rest of the animals (Group Ib five rabbits) were feeding orally with 4×103 E.hisotlytica cysts to study the effect of immunization with K- antigen .Ten animals second group (Group II) was feeding orally by E.histolytica (positive control) to determine the infection dose and negative group (Group III) was feeding by (a physiological (saline) solution).The study showed inhibition of k-antigen for severs histological changes in the intestine following parasitic infection compared with positive control. We suggest the use k-antigen of K.pneumoniae as immunizer against infections with E.histolytica.
Keywords | Klebsiella, K-antigen, Entamoeba histolytica
Received | July 14, 2020; Accepted | July 22, 2020; Published | September 12, 2020
*Correspondence | Karama Al-Taee, Lecturer Department of Basic sciences, College of Dentistry, University of Anbar; Email: [email protected]
Citation | Al-Taee KTA, Al-Dulaimi KK (2020). Study the histological changes in intestine of infected rabbits with entamoeba histolytica after immunization with klebsiella pneumonia k-antigen. Adv. Anim. Vet. Sci. 8(11): 1250-1255.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.11.1250.1255
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Al-Taee and Al-Dulaimi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
About 50 million people world-wide have been infected with Entamoeba histolytica causing a mortality rate of over 100,000 per year (Tan et al., 2010). Entamoeba histolytica is a parasite which is traced back to protozoan that infects humans and causes the disease amebiasis (Solaymani and Petri., 2008).The inability to develop an active immunizing against parasites that infected human belongs to the fact that the parasite biology is far more complex than other microbes (Astronomo and Burton, 2010). Klebsiella pneumonia is (a common hospital-acquired pathogen), causing, nosocomial pneumonia, urinary tract infections, Operational wound infections and blood infections (Lundberg et al., 2013). Immunization based on surface polysaccharides, O-antigens (lipopolysaccharide, LPS) and K-antigens (capsule polysaccharide, CPS) present in K. pneumonia is highly appropriate as a vaccine , it has been sophisticated and reached a stage I trials in humans (Follador et al., 2016).
.
Vaccination with outer membrane proteins purified from K. pneumoniae (OmpK17 and OmpK36) trigger defenses against Klebsiella pneumoniae in the mice infection model (Hussien et al., 2018). Immunization based on carbohydrate antigens is an applicable choice for parasite vaccine development as in the success of vaccines of carbohydrate to avoid infections caused by bacteria. It is confirmed that the response immunity against the capsular polysaccharide gives defense against the disease. The immunological Characteristics of bacterial polysaccharide became the goal of a lot of examiners. Today, many vaccines depend on purified surface polysaccharide (K-antigen) or on neoglycoconjugates (Weintraub, 2003). The host’s immune system can distinguish pathogenic microbial polysaccharides and stimulate the production of polysaccharides- specific antibodies to give protection against microbes (Astronomo and Burton, 2010). The bacterial microbiota in the intestine has lately been shown to be necessary in adjust (bone marrow) operation that lead to immune effector cells essential to get rid of pathogens, such as neutrophils and inflammatory macrophages, and supply with defenses against infection caused by enteric bacteria (Burgess and Petri., 2016). Polysaccharide purified from k.pneumoniae proved safe for use as vaccines and therapies for protection and treatment disease caused by k.pneumoniae (Cryzy et al., 1986; C. Opoku-Temeng et al., 2019).Therefore, our study suggests the use of polysaccharide extracted from K.pneumoniae as a vaccine against infection with E.histolytica.
MATERIAL and METHODS
The samples of Bacteria
K. pneumoniae was gained from the Laboratory of microbiology at the college of Sciences/ University of Baghdad. The identification was conducted by using Api-20E. By using the BHI (Brain- Heart Infusion agar), bacteria were cultured. The existence of the (k- antigen) capsule is done according to the method (Atlas., 1995) and the exopolysaccharides extracted the capsule according to the method put up by (Domenico et al., 1989). The sugar was detected by Molisch test, according to (Elzagheid, 2018) and the quantity of sugar isolated from the bacterial capsule extracted by carrying out the process of (phenol-sulfuric acid) described by (Han, Pei-pei, et al., 2019).
The Parasites Samples
The sample of E.histolytica was gained from the out patient 30 years female that attended to Al-Yarmouk Teaching Hospital, who infected with severe bloody diarrhea, samples were tested by the wet preparation technique. The (E.histolytica was isolated as stated by the procedure of (Garcia and Bruckner, 1997). The cysts were collected by sugar solution (dextrose) flotation technique as described by (Hassan, Hala Abdalazim, et al., 2019). The parasite cysts were then counted according to the method described by (Clark & Diamond, 2002).
The Rabbits of Experiment
In our study, thirty adult white rabbits (New Zealand males) were gained from the National Center for Drug Control and Research. In the experiment, rabbits were used that weighs ranging from 1200 to 2000 g and its age) from 12 to 20 months. The laboratory animals were kept in animal cages of the department of biology/College of Science/ University of Baghdad, and fed with special food prepared from the pharmacy department of the Samarra Pharmaceutical Laboratory. All of the experiments (injection and dissection) to the animals were obtained permission from the National Center for Drug Control and Research.
Detection the Infectious Dose
The contagious dose is more than 1×103 cysts (Ryan and Ray, 2004). Therefor the dose required for infection was determined by preparing four doses of cyst in 0.1 ml of sugar solution (dextrose) began from 2×103 cysts. Eight animals were fed orally by, 2×103, 3×103, 4×103 and 5×103 (cysts per dose) and two animals fed with 0.1 ml of sugar solution as negative control. After two weeks samples of stool were examined under light microscope (Heymann, 2008). Diarrhea and loss of appetite were observed in animals that feed with 4×103, 5×103 (cysts per dose). Upon examination of the stool, numbers of parasite cysts were found in 4×103, 5×103 cysts per dose; therefore 4×103 was adopted as a feeding dose to cause infection. This group considers as positive group (Group II) Animals feeding with 4×103 were dissected and specimens of the intestinal tissue were taken.
K-antigen Concentration
The antigen concentrations were prepared by dissolving the antigen powder in distilled water the concentrations were (0, 10, 20, 40,100) mg/ ml.
Design of the Study
After confirming the health of the thirty white adult male rabbit and that they do not have any apparent diseases the animals were divided into 3 groups Table 1. The Group I comprises ten animals that were injected subcutaneous and intramuscular by 1 ml of (0, 10, 20, 40,100) mg/ ml K antigen per animal. After the immunization period (two weeks) the Group I divided into two Groups. Group Ia and Group Ib. Five animals Group Ia were dissected, while the other five animals Group Ib were fed by the (cysts) E.histolytica (0.1) ml of 4×103 cyst / dose for every rabbit. To detect the infection with the parasite, animal stool was tested daily. The Group II that was fed orally by E.histolytica (0.1) ml of (2×103, 3×103, 4×10 3 5×103 cyst /dose) for each animal as described in (detection the infectious dose). Animals were dissected after two weeks at the end of each experimental animals. The third group ten animals (Group III) was the control group. Its animals were given a (physiological) saline solution) for the period of the tests a dosage of (2) ml every day for two weeks.
The Histological Sections
After two weeks the rabbits were dissected in Group I and Group II Table1 and specimens of the intestinal tissue (0.5 cm3) were set in (10%) (formalin) for 12 hours after that
Table 1: Design of the study, show division of experimental animals.
|
No. of group |
No. of animals | Sub-group |
|
|
Group I |
10 Test group/injected with((0, 10, 20, 40,100) mg/ ml) K antigen |
Group Ia 5 |
Dissected after two weeks
|
| Group Ib5 |
Fed orally with the (4×10 3 cysts) E.histolytica |
||
|
Group II |
10 |
Group IIa8
|
Positive group/fed with cysts 2×103, 3×103, 4×10 3 5×103 cyst /dose |
|
Group IIb2 |
Control /fed with sugar(dextrose) solution | ||
|
Group III |
10 | - |
Control group/ fed with saline solution |
specimens washed with water for 10 minutes . All sections were dried in graded ethanol, cleared in xylene before embedding them in paraffin (Suzuki et al., 2012). 4-5 μm the samples of tissue were cut. By alcohol 30% concentration the sections were fixed and then placed on glass slides to be ready for staining with hematoxylin and Eosin (Bancroft et al., 1982). By the compound light microscope the section of tissue were examined in laboratory of Histology at the college of Sciences/ University of Baghdad.
RESULTS & DIScussion
There is a great similarity between the digestive system of rabbits and humans, so rabbits can be considered a good model for the study of gastrointestinal diseases (Kararli, 1995). After 10 days the control rabbits group (Group III) which was given a (physiological saline solution) for the period of the experiment was characterized by normal weight and normal appearance of the histological sections in small intestine and large intestine Figures (1a, 2a, 3a & 4a). The features of intestine were normal microvilli as well as the natural appearance of the mucosa goblet cells compared with animal in positive control which refuse food after 10 days and show decrease in weight. The sections of the tissue that obtained from intestine animals infected with the E.histolytica group (Group IIa) appeared many changes in the small intestine and large intestine Figures (1b, 2b, 3b & 4b), Cecal lesion and infiltration of inflammatory cells, mucosal ulceration, sever destruction in intestinal microvilli, hyperplasia in goblet cells and intestinal mucosal degeneration in colon section. These results were in agreement with (AL-Nafouli., 2004). The section also showed sloughing in epithelial cells and presence of trophozoit of E.histolytica and this is in agreement with the results by (Chadee and Meerovitch, 1985).
The histological sections of K-antigen group show hyperplasia and increases in length of intestinal microvilli in the duodenum (Fig. 1c). In ileum there is apoptosis in epithelial cell lining microvilli and increase in length of intestinal microvilli (Fig. 3c) .The section in jejunum showed hyperplasia in mucosa and increased thickness of jejunum’s walls (Fig. 2c). These changes are due to the immune response to the (capsular antigen) given to the animals. (AL-Kabi., 2006) was pointed to these results. The rabbits can be immunized by used the protein of the outer membrane and the capsule of K. pneumonia.
The results of a study achieved by (Cryz et al., 1985) showed increases in IgG and IgM titers after immunization with capsulated polysaccharide. The IgG antibodies obtained from immune serum was given a significant effect in preventing deadly burn wound sepsis in experimental mice due to K. pneumoniae K1.
The histological sections of the Group Ia treated with (K-Antigen) showed simple histological changes Figures ( 1c, 2c, 3c & 4c). The appearance of the colon is close to normal, and also having normal of mucosa except the hyperplasia (Fig. 4c). The changes in jejunum show increases in goblet cells and increase in length of mucosa region lining jejunum (Fig. 2c).
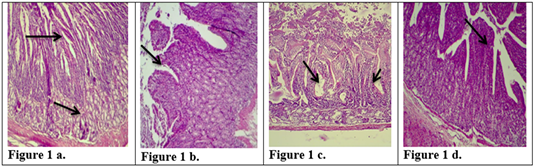
Figure 1: Description of histological changes in duodenum sections. a): Group III, negative control, animal feeding with saline solution. Normal appearance of microvilli, Brunner’s glands in duodenum. (200 X),(H & E)). b): Group II, positive control, animal feeding with E.histolytica cyst, hyperplasia and increases in microvilli and mucosal glands of duodenum. (200 X),(H & E). c): Group I a, section in the animal duodenum immunized by K-antigen, showing hyperplasia increases in length of intestinal microvilli (200 X),(H & E) d): Group I b, section in the duodenum shows the presence of hypoplasia of the intestinal microvilli with an increase in the mucous glands after infected immunized animal with E.histolytica (200 X)(H & E).
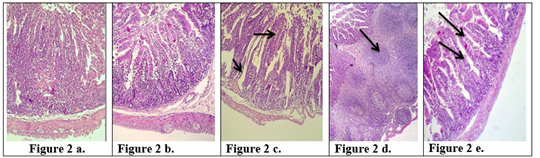
Figure 2: Description of histological changes in the jejunum sections. a): Group III, negative control, (200 X) (H & E). b): Group II, positive control, hyperplasia, (200 X) (H & E). c): Group I a, the section in jejunum shows increases in goblet cells and increases in length of mucosa region lining jejunum after an immunized animal with K-antigen (200 X) (H & E). d): Group I b, section in jejunum shows hyperplasia in the lymphatic glands (peyer’s patch) L.N. of submucosa with germinal center this refers to induce immunity (100 X) (H & E). e): Group I b, section in jejunum after infection with E.histolytica shows simple degenerations in the cells surrounding microvilli with filtration of inflammatory cells inside the cavity of intestinal microvilli and decrease in goblet cells (200 X)(H & E).
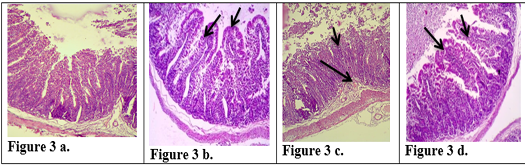
Figure 3: Description of histological changes in ileum sections. a): Group III, negative control animal feeding with saline solution, normal appearance of ileum (200 X)(H & E). b): Group II, positive control, animal feeding with E.histolytica cyst, (200 X)(H & E). c): Group I a, after injection with k-antigen the section in the ileum shows increases in length of intestinal microvilli and apoptosis in epithelial cell lining microvilli (200 X)(H & E). d): Group I b, section in jejunum after infection with E.histolytica shows simple degenerations in the cells surrounding microvilli with filtration of inflammatory cells inside the cavity of intestinal microvilli and decrease in goblet cells (200 X) (H & E)
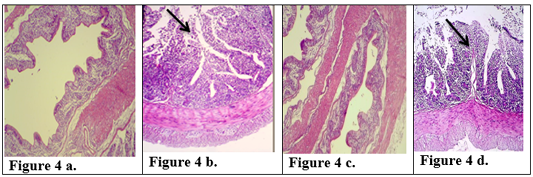
Figure 4: Description of histological changes in colon sections. a): Group III, negative control, normal appearance of colon (200 X) (H & E). b): Group II, positive control.(200 X) (H & E) submucosal ulceration. c): Group Ia ,section in the colon looks like normal and there is no hyperplasia in lining of colon mucosa after immunized animal with K-antigen (200 X) (H & E) d): Group I b, colon section shows superficial mucosal ulceration with heavy filtration of inflammation cells in and between folded intestinal microvilli after infected immunized animal with E.histolytica (200 X) (H & E).
The Group Ib infected with E.histolytica after immunization with K- antigen did not show severe histological changes (Fig 1d, 2d, 2e, 3d, 4d) compared with positive control. The Section in jejunum showed simple degenerations in the cells surrounding microvilli with filtration of inflammatory cells inside the cavity of intestinal microvilli (Fig. 2e) shows hyperplasia in lymphatic glands (peyer’s patch) L.N. of submucosa with germinal center (Fig 2d) and this result points to induced immunity. Colon section showed superficial mucosal ulceration with heavy filtration of inflammation cells in and between folded intestinal microvilli and increase in goblet cells (Fig 4d). Compared with positive control which showed submucosal ulceration (Fig 4b), this increase in goblet cells leads to produce mucus, which is a protective layer against pathogen invasiveness. Resistance to damage to the laboratory animals intestine that infected with parasite may be due to the fact that the polysaccharide injected into the rabbits has produced an immune response that prevented the damage caused by E. histolytica.These results are agree with the study carried out by (Al-Kabi, 2006). Which was pointed in his study to increase the phagocytic cells after immunization with lipopolysaccharide, which is consistent with the results of (AL-Taei, 1996) who realized increasing the phagocytic process rate in (rats) immunized with (polysaccharides) isolated from (Rhizobium leguminosarum) before and after infected by cysts of (Echinococcus granulosus). A study by (Houpt et al., 2004) indicated that immunization with the Gal/GalNAc lectin can protect mouse intestine from amebiasis. (Gilchrist et al., 2015) found that fecal IgA was correlated with prevent recurrent infection with E. histolytica. Study by (Burgess et al., 2014) suggested that colonization of the gut with the bacteria (commensal Clostridia) was protective during infection with E. histolytica. Another study was shown that the polysaccharide processed and presented on MHC II) like the processing of proteins ,to activate CD4+ (helper T-cells) and stimulation of( immune responses) dependent to (T-cell) (Cobb et al., 2004). Our study suggested that immunization with capsulated polysaccharide k-antigen isolated from k.pneumoniae may be used as immunizer against infection with E.histolytica. Research has proved its safe use as a vaccine in humans against infection with k.pneumoniae (Opoku-Temeng et al., 2019).
CONCLUSIONS
Polysaccharide k-antigen isolated from k.pneumoniae may be used as immunizer against infections with E.histolytica parasite.
Acknowledgements
We have taken efforts in this project. However, it would not have been possible without the kind support and help of many individuals and organizations. I would like to extend my sincere thanks to all of them, especially the Laboratory of microbiology in the college of Sciences / University of Baghdad, Al-Yarmouk Teaching Hospital, and pharmacy department of the Samarra /Pharmaceutical Laboratory for their help in completing this research.
conflict of interest
The authors have no competing interests.
Authors contribution
All authors conceived and designed the study. Khadija Khleaf Abdulla AL-Dulaimi conducted the experiments,analyzed the data .KaramaTahreer Ahmed AL-Taee wrote the paper .All Authors contributed to manuscript revisions.All authors approvedthe final version of the manuscript and agree to be held accountable for the content therein.
REFERENCES






