Advances in Animal and Veterinary Sciences
Research Article
Impact of Using Different Sources and Levels of β-Glucan and Mannan Oligosaccharide on Performance Traits of Broiler Chicks
Mamdouh Omar Abd-Elsamee1, Abd-Elhakim Saad Abd-Elhakim2, Ragab Rezk Elsharkawy2*, Hany Mohamed Ramadan Elsherif1
1Department of Animal Production, Faculty of Agriculture, Cairo University, Giza, Egypt; 2Animal Production Research Institute, Agricultural Research Center, Giza, Egypt.
Abstract | This study compared between yeast cell wall (YCW) and mushroom (MR) as sources of both β-Glucan and mannan oligosaccharide (β-G+MOS) at different supplementation levels on growth performance, carcass characteristics and plasma protein fractions of 420 unsexed a day-old Arbor Acres broiler chick. Chicks were randomly allocated to seven dietary treatments each of 4 replicates (15 birds per replicate). Birds had the same management protocol and were housed in three-deck batteries, and had free access to feed and water up to 35 days of age. Birds were switched to the experimental diets as follows; T1, a basal control diet. Diets T2, T3 and T4 were supplemented with a combination of β-G+MOS at 100, 200 and 300 ppm from YCW, respectively. Diets T5, T6 and T7 were were supplemented with a combination of β-G+MOS at 100, 200 and 300 ppm from MR, respectively. Results show that live body weight (LBW), live body weight gain (LBWG), feed consumption (FC) and feed conversion ratio (FCR) were not significantly affected by source or level of β-G+MOS during all experimental periods, except that YCW had higher (p=0.02) LBWG during finisher and lower FC during starter (p=0.001) than MR based diets. Dietary addition of β-G+MOS improved final LBWG (p=0.007) and FCR (p=0.005) compared to the control. Broilers group fed 200 ppm β-G+MOS from YCW recorded more live weight gain by 13.2 percent and better FCR by 12.17 percent over the control. Carcass percent of 300 ppm β-G+MOS from YCW group was the highest carcass (p=0.03) and being higher by 4.52 percent over the control. It could be suggested that supplementing of β-G+MOS to broiler diets might improve broiler performance regardless the sources or level although, YCW source may be superior especially at finisher period.
Keywords | Beta glucan (β-G), Mannan oligosaccharide (MOS), yeast cell wall (YCW), mushroom (MR), broiler performance
Received | June 01, 2021; Accepted | August 07, 2021; Published | September 25, 2021
*Correspondence | Ragab Rezk Elsharkawy, Animal Production Research Institute, Agricultural Research Center, Giza, Egypt; Email: [email protected]
Citation | Abd-Elsamee MO, Abd-Elhakim AS , Elsharkawy RR, Elsherif HMR (2021). Impact of using different sources and levels of β-glucan and mannan oligosaccharide on performance traits of broiler chicks. Adv. Anim. Vet. Sci. 9(11): 1851-1862.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1851.1862
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abd-Elsamee et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The poultry production is one of the biggest sectors in livestock production, it covering animal protein demanding for millions of people world wide, because poultry meat is cheaper and healthier than red meat (Farrell, 2013). Broiler chick’s selection for commercial goals has been extremely developed over the recent decades (Tallentire et al., 2016). It can be a result of feed conversion efficiency increasing and a reduction of feed costs (Taha, 2003). So, feed alternatives needed to improve broiler growth performance and protect the birds from disease infections.
Many countries has been used antibiotics as growth promotant, as birds during their breeding are exposed to many stressors that could effect on health, growth and mortality. Also, they have been used as immuno-modulators, hence participate to enhance the growth performance and health status of birds (Cheng, 2014; Fadl et al., 2020). Although, there are increasingly consumer concerns about drug residues in meat products and the rise of antibiotic resistance of pathogenic bacteria (Gadde et al., 2017; Wang et al., 2018). For these reasons, the European Union, besides many countries banned the use of antibiotics in animal diets as growth promotants since 2006 (Toghyani et al., 2011; Khan and Naz, 2013; Abudabos et al., 2016; Zia-Ur-Rehman et al., 2017). Otherwise this ban has resulted in a negative impact in animal health and growth performance (Castanon, 2007; Waqas et al., 2019). So, there is a continuous seek for safe alternatives serve the same goals achieved by antibiotics (Gao et al., 2008; Brummer et al., 2010; Seal et al., 2013; Khan et al., 2016; Rahman et al., 2017; Abudabos et al., 2018).
Prebiotics defined as non-digestible ingredients that affect the host by stimulating the growth of beneficial bacteria in the gastro-intestinal of the host (Gibson and Roberfroid, 1995; Kolida and Gibson, 2011; Obolewska et al., 2017). Beta-glucans (β-G) and mannan oligosaccharides (MOS) are prebiotics commonly used in many poultry species (Raa, 1996; Song et al., 2014). MOS and β-G are carbohydrates, which form the structure of cell wall of yeast, fungi, algae, and cereal grains (Lipke and Ovalle, 1998; Yang et al., 2009; Vetvicka and Vetvickova, 2015). The YCW MOS and β-G have beneficial effects on poultry gut health and growth performance (Spring et al., 2000; Shao et al., 2013; Lourenço et al., 2016; Rizwan et al., 2016). Yeast β-G supplementation can protect poultry against many pathogens such as Salmonella, E.coli and Eimeria species by improving immune response and gut health (Lowry et al., 2005; Revolledo et al., 2009; Huff et al., 2010). Cox et al. (2010a) showed that supplementation of β-glucan at 100 ppm in broiler chicken diets maintain gut health by reducing the intestinal damage severity of broiler chickens challenged with Eimeria. Shao et al. (2013) concluded that supplementation of yeast β-G at 100 ppm in diets of broiler chickens challenged with Salmonella typhimurium can improve gut health due to protect intestinal mucosal barrier against salmonella and increase villi heights compared to un-supplemented challenged chicks. Tian et al. (2016) reported that supplementation of yeast β-G at 200 ppm in Clostridium perfringens challenged broiler chicken diets improved growth performance accompanied with increasing up to 10% in average daily gain whereas decreasing up to 6% in feed conversion ratio (FCR) compared to control challenged chicks. Addition of MOS has been heavily investigated to assess their potential as alternatives to antibiotic growth promoters (Spring et al., 2000; Bozkurt et al., 2012; Sohail et al., 2012; Xiao et al., 2012; Attia et al., 2014). Jahanian et al. (2016) found that dietary MOS supplementation at 2000 ppm in aflatoxin-challenged broiler chicks diet enhanced growth performance via improving feed digestibility and increase nutrient absorptive surface. Jahanian and Ashnagar (2015) and Ghasemian and Jahanian (2016) showed that addition of MOS at 1000-1500 ppm to laying hen diets enhanced growth performance due to improve gut health and nutrients digestibility under challenged conditions.
Therefore, this study was conducted to investigate the effect of dietary MOS and β-G from yeast cell wall or edible mushroom on growth performance and immune status in broiler chickens.
MATERIALS AND METHODS
The present experiment was designed and approved by institutional animal care and use committee (CU-IACUC) at Cairo University, Egypt (CU-II-F-8-21).
Experimental design and management
This experiment was carried out at El-Azab Research Farm, Fayoum, Egypt during December 2019 to January 2020. Experimental diets and water were offered ad-libitum throughout the growth trail term. Chicks in all treatments were kept under similar management conditions. Artificial lighting was provided all over 24 hours during the whole experimental period. Gas heaters were used to provide chicks with heat needed for brooding.
Chicks were vaccinated against Newcastle disease (ND) with hichner B-1 (0.2 cm subcutaneous injection) + IB (eye drop) on the third day of age. Chicks were vaccinated twice against infectious bursal disease (IBD) in drinking water firstly on the 8th day of age and the second was on the 14th day of age. Chicks were also vaccinated against ND+AI (0.5 cm sub cutaneous injection) and against ND+IB (eye dropping) on the 10th of the age.
β-G+MOS sources
Mushroom (Pleurotus ostreatus): Dried and grinded edible mushroom was purchased from the Egyptian local market, it containing 54% β-glucan + 5% MOS. According to Synytsya et al. (2009), the mushroom fruit body is about 5–15% of dry matter containing 19–35% crude protein.
Fubon yeast cell wall (saccharomyces cerevisiae)
A commercial source cell wall extract of saccharomyces cerevisiae contained 20% β-G+20% MOS was provided by Angel yeast co., ltd– china. It composed of 15-30% of the dry weight of vegetative yeast (Orlean, 1997). Commercial YCW contains 30-60% polysaccharides including mannan and β-glucan polymers, 15-30% proteins, 5-20% lipids, and a small portion of chitin (ERUASYP, 2015).
Experimental birds and diets
A total of 420 unsexed day-old Arbor Acres broiler chicks with 39.2 g initial weight were housed in 3 deck batteries with cages dimensions 60*100*25 cm3. Birds were divided into seven dietary treatments with 4 replicates of 15 birds each and were kept in a semi closed house under the same experimental conditions. Diets were formulated to meet the nutrients requirements of Arbor Acres (2019) in a mash form as indicated in Table 1. Dietary treatments were the control (T1), groups 2 to 4 were fed diets supplemented with 100, 200 and 300 ppm β-G and MOS from yeast cell wall, respectively, and groups 5 to 7 were fed diets supplemented with 100, 200 and 300 ppm β-G and MOS from mushroom, respectively.
Table 1: Ingredients composition and calculated chemical analysis of the basal diets.
| Ingredient | Starter | Grower | Finisher |
| 1-14 d | 15-28 d | 29-35 d | |
| Yellow corn | 54.30 | 57.12 | 61.50 |
|
Soybean meal (46%) |
34.30 | 30.21 | 24.58 |
|
Corn gluten (60%) |
4.50 | 4.69 | 5.10 |
| Crude soy oil | 2.43 | 3.50 | 4.35 |
| Limestone | 1.50 | 1.58 | 1.58 |
| Mono-calcium phosphate | 1.50 | 1.48 | 1.48 |
| Vitamins and Minerals Premix | 0.3 | 0.3 | 0.3 |
| NaCl | 0.32 | 0.26 | 0.26 |
| Sodium bicarbonate | 0.1 | 0.17 | 0.17 |
| DL-Methionine | 0.24 | 0.16 | 0.16 |
| L-Lysine HCl | 0.18 | 0.22 | 0.22 |
| Choline chloride (60%) | 0.33 | 0.31 | 0.30 |
| Total | 100 | 100 | 100 |
| Calculated analysis | |||
| Crude protein (%) | 23.00 | 21.50 | 19.50 |
| Metabolizable Energy (kcal/kg) | 3000 | 3100 | 3200 |
| Crude Fiber (%) | 3.25 | 3.09 | 2.89 |
| Ether Extract (%) | 5.07 | 6.19 | 7.14 |
| Calcium (%) | 0.91 | 0.92 | 0.90 |
| Available Phosphorus (%) | 0.47 | 0.46 | 0.45 |
| Methionine | 0.65 | 0.55 | 0.53 |
| Methionine+Cystine | 1.03 | 0.90 | 0.85 |
| Lysine | 1.34 | 1.26 | 1.10 |
| Sodium | 0.17 | 0.16 | 0.16 |
Each 1 Kg diet contains: Vit. A 12000 IU, Vit. D 5000 IU, Vit. E 80 IU, Vit. K3 3.20 mg, Vit. B1 3.20 mg, Vit. B2 8.60 mg, Vit. B12 0.017 mg, Vit. B6 4.30 mg, Niacin 65 mg, Pantothenic acid 20 mg, Folic acid 2.20 mg, Biotin 0.22 mg, Copper 16 mg, Iodine 1.25 mg, Iron 20 mg, Zinc 110 mg, Manganese 120 mg, Selenium 0.30 mg and Cobalt 0.10 mg. *The 100, 200 and 300 ppm of β-G+MOS from YCW corresponded to 25, 50 and 75-gram YCW per 100 kg diet for starter, grower and finisher diets, respectively. *The 100, 200 and 300 ppm of β-G+MOS from MR corresponded to16.9, 33.8 and 50.7 gram MR per 100 kg diet for starter, grower and finisher diets, respectively.
Measurements
Growth performance: Chicks were individually weighed at the beginning and at the end of each growth interval to calculate live body weight gain (LBWG). Feed consumption (FC) of each period was recorded/bird/pen and used to calculate the amount of feed consumed (kg) to produce 1 kg of meat.
Carcass characteristics: Five birds of each group representing the average final treatment weight ±10% were overnight fasted then were slaughtered to complete bleeding, and plucked of feathers. The carcass, lymphoid organs (bursa, thymus and spleen), and giblets (heart, gizzard and liver) were weighed and expressed as percent of live body weight.
Blood parameters: At slaughtering, birds assigned to slaughter test were also used for blood plasma assay. Blood samples were immediately collected from the same slaughtered birds (5 birds/ treatment) into dry clean centrifuge tubes containing droplets of heparin solution and plasma was separated by centrifugation at 3000 r.p.m. for 15 minutes. Collected plasma was kept at -20°C at deep freezer for subsequent metabolites determinations. Total protein (Tp; g/dL) according to Gornal et al. (1949), and albumin (A; g/dL) according to Doumas et al. (1971) were measured. Globulin (G; g/dL) was calculated by the difference between Tp and A, and A/G ratio was subsequently calculated.
Statistical analysis
The statistical analysis was computed using analysis of variance using SAS program (SAS® Institute, 2004). The significance differences means between treatments were separated using Duncan’s Multiple Range (Duncan, 1955).
The statistical models used were:
One way analysis: Yij= µ + Ti + eij, where Yij= an observation, µ= the overall mean, Ti= Effect of treatment (i= 1, ...7) and eij= experimental error.
Factorial analysis: Yijk = µ + Si + Lj + SLij + e ijk, where Yij = an observation, µ= overall mean, Si= β-G+MOS source effect (i = 1, 2), Lj= β-G+MOS level effect (j = 1, 2, 3), SLij = effect of interaction between β-G+MOS source and level, and eijkl= experimental error.
Results and Discussion
Productive performance
Effect of different β-G+MOS sources, levels and their interactions on broiler performance; live body weight (LBW), average body weight gain (ABWG), feed consumption (FC) and feed conversion ratio (FCR) at starter, grower and finisher periods are presented in Tables 2 and 3. Broiler performance did not significantly affected by either β-G+MOS sources or levels during all experimental periods and overall period except BWG was significantly increased at finisher period and FC was significantly decreased at grower period with YCW vs. mushroom (MR) source. Chicks fed diets with added β-G+MOS were significantly improved BWG and FCR compared with those fed control diet at overall period. Generally, the group of (T6) which fed 200 ppm β-G+MOS from MR was significantly (P= 0.045) recorded the highest ABWG during starter period by 12.36% compared to control and numerically during grower period. Group fed 200 ppm β-G+MOS of YCW (T3) was significantly achieved the highest BWG during overall period by 13.22% and best FCR by 12.17% compared to control.
Lymphoid organs
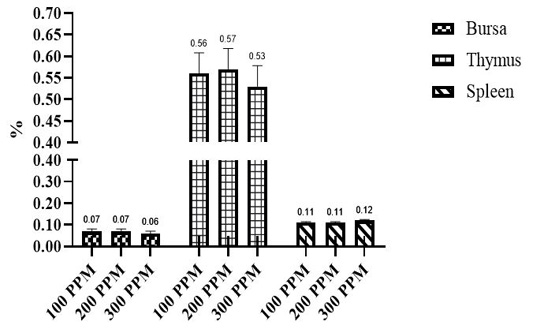
Effects of different β-G+MOS sources, levels and their interactions on relative lymphoid organs percent are in Table 5 and Figures 1 and 2. Chicks fed diets added with β-G+MOS from YCW were significantly (P= 0.02) recorded thymus percent higher than MR groups. Otherwise no significant differences were observed in relative lymphoid organs percent due to neither β-G+MOS levels nor treatments.
Plasma protein parameters
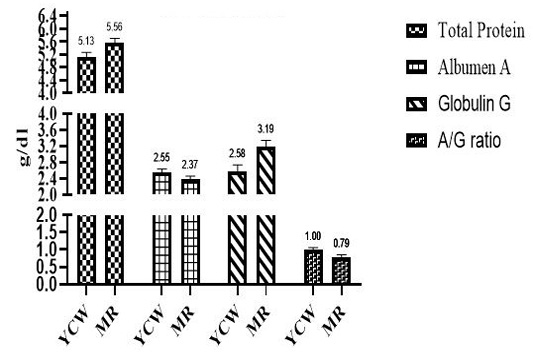
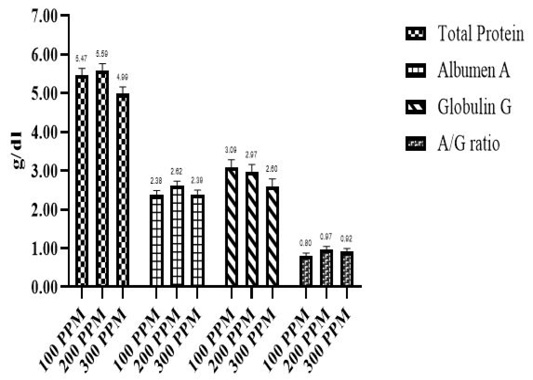
Effect of different β-G+MOS sources, levels and their interactions on blood plasma protein parameters; (Total protein (Tp), albumin (A) and globulin (G)) is presented in Table 5 and Figures 3 and 4. Chicks fed MR β-G+MOS source were significantly (P=0.02) recorded higher Tp, also G value was significantly (P=0.01) increased in chicks groups fed MR β-G+MOS compared to those fed YCW source. While lower A/G ratio was significantly (P=0.03) recorded for chicks fed MR β-G+MOS than those fed YCW source, where no significant differences were detected with β-G+MOS levels in all plasma protein parameters except Tp values. Chicks fed 200 ppm was significantly (P= 0.04) recorded higher Tp than those fed 300 ppm. All blood plasma protein parameters were significantly affected by β-G+MOS either sources and levels interactions or control group. Generally, the blood plasma protein parameters values were within normal range.
Table 2: Effect of β-G+MOS source, level and treatments on live body weight and live body weight gain.
|
* β-G+MOS sources |
β-G+MOS levels (ppm) |
Live body weight (g) | Live body weight gain (g) | |||||||||||||
| Starter | Grower | Finisher | Overall period | |||||||||||||
| 1d | 14d | 28d | 35d | 1-14d | 15-28d | 29-35d | 1-35d | |||||||||
| Source: | ||||||||||||||||
| YCW | - | 39.4 | 357.5 | 1317.4 | 1919.8 | 318.0 | 959.9 |
602.3a |
1880.3 | |||||||
| MR | - | 38.2 | 365.6 | 1340.0 | 1901.2 | 326.4 | 974.3 |
561.1b |
1862.0 | |||||||
| SEM | - | 0.216 | 4.12 | 17.56 | 23.32 | 4.015 | 15.98 | 11.80 | 23.23 | |||||||
|
P value |
- | 0.37 | 0.18 | 0.37 | 0.58 | 0.15 | 0.53 | 0.02 | 0.58 | |||||||
| Level: | ||||||||||||||||
| - | 100 | 39.7 | 356.0 | 1300.2 | 1883.3 | 316.3 | 944.1 | 583.1 | 1843.6 | |||||||
| - | 200 | 39.4 | 369.7 | 1358.3 | 1926.3 | 330.3 | 988.5 | 568.0 | 1886.8 | |||||||
| - | 300 | 38.9 | 358.8 | 1327.6 | 1921.8 | 319.9 | 968.8 | 594.1 | 1882.9 | |||||||
| SEM | - | 0.264 | 5.05 | 21.51 | 28.57 | 4.91 | 19.57 | 14.45 | 28.46 | |||||||
|
P value |
- | 0.12 | 0.15 | 0.19 | 0.51 | 0.14 | 0.29 | 0.45 | 0.50 | |||||||
| Treatment: | ||||||||||||||||
| Control T1 | - | 38.4 |
339.3 c |
1267.2 |
1714.3b |
300.8b |
927.9 |
447.1c |
1675.9b |
|||||||
| YCW T2 | 100 | 39.8 |
352.7 bc |
1311.7 |
1910.0a |
312.9b |
958.9 |
598.3ab |
1870.2a |
|||||||
| YCW T3 | 200 | 39.7 |
362.4ab |
1346.4 |
1937.3a |
322.6ab |
984.0 |
590.9ab |
1897.6a |
|||||||
| YCW T4 | 300 | 38.9 |
357.3abc |
1294.3 |
1912.0a |
318.4ab |
936.9 |
617.7a |
1873.0a |
|||||||
| MR T5 | 100 | 39.5 |
359.4abc |
1288.8 |
1856.7a |
319.8ab |
929.4 |
567.9ab |
1817.1a |
|||||||
| MR T6 | 200 | 39.1 |
377.1a |
1370.2 |
1915.2a |
338.0a |
993.0 |
545.0b |
1876.1a |
|||||||
| MR T7 | 300 | 38.9 |
360.3abc |
1361.0 |
1931.6a |
321.4ab |
1000.6 |
570.6ab |
1892.7a |
|||||||
| SEM | - | 0.387 | 6.97 | 28.50 | 39.05 | 6.85 | 26.33 | 21.54 | 38.90 | |||||||
|
P value |
- | 0.148 | 0.045 | 0.123 | 0.0075 | 0.045 | 0.263 | 0.0004 | 0.0075 | |||||||
a, b means in each column, within each factor, bearing the same superscripts are not significantly different. *β-G+MOS= β-Glucan + Mannan oligosaccharides, YCW= Yeast Cell Wall, MR= Mushroom and D= Day.
Table 3: Effect of β-G+MOS source, level and treatments on feed consumption and feed conversion ratio.
|
β-G+MOS sources |
β-G+MOS levels (ppm) |
Feed consumption (g) | Feed conversion ratio (g feed/g gain) | ||||||
| starter | grower | finisher | overall period | starter | grower | finisher | overall period | ||
| 1-14 d | 15-28 d | 28-35d | 1-35d | 1-14d | 15-28d | 28-35d | 1-35d | ||
| Source | |||||||||
| YCW | - | 367.3 |
1328.3b |
910.6 | 2606.3 | 1.15 | 1.38 | 1.51 | 1.38 |
| MR | - | 368.8 |
1386.5a |
910.1 | 2665.4 | 1.13 | 1.42 | 1.62 | 1.43 |
| SEM | - | 4.76 | 6.33 | 18.63 | 22.34 | 0.018 | 0.021 | 0.042 | 0.018 |
|
P value |
- | 0.82 | 0.0001 | 0.98 | 0.07 | 0.34 | 0.22 | 0.08 | 0.10 |
| Level | |||||||||
| - | 100 | 357.7 | 1341.8 | 897.2 | 2596.8 | 1.13 | 1.42 | 1.54 | 1.41 |
| - | 200 | 371.6 | 1363.7 | 909.7 | 2645.1 | 1.12 | 1.38 | 1.61 | 1.40 |
| - | 300 | 374.8 | 1366.7 | 924.0 | 2665.6 | 1.17 | 1.41 | 1.56 | 1.41 |
| SEM | - | 5.83 | 7.75 | 22.82 | 27.36 | 0.022 | 0.026 | 0.052 | 0.022 |
|
P value |
- | 0.11 | 0.07 | 0.71 | 0.21 | 0.24 | 0.45 | 0.66 | 0.91 |
| Treatment | |||||||||
| Control T1 | - |
387.8a |
1330.4cd |
900.69 | 2618.9 |
1.29b |
1.43 |
2.03b |
1.56b |
| YCW T2 | 100 |
369.4abc |
1327.2cd |
907.91 | 2604.6 |
1.18ab |
1.39 |
1.52a |
1.39a |
| YCW T3 | 200 |
359.9bc |
1312.9d |
931.25 | 2604.1 |
1.11a |
1.33 |
1.57a |
1.37a |
| YCW T4 | 300 |
372.6ab |
1344.8cd |
892.75 | 2610.1 |
1.17ab |
1.43 |
1.44a |
1.39a |
| MR T5 | 100 |
346.0c |
1356.4bc |
886.66 | 2589.1 |
1.08a |
1.46 |
1.56a |
1.42a |
| MR T6 | 200 |
383.3ab |
1414.5a |
888.25 | 2686.1 |
1.13a |
1.42 |
1.64a |
1.43a |
| MR T7 | 300 |
377.1ab |
1388.6ab |
955.41 | 2721.1 |
1.17a |
1.39 |
1.68a |
1.43a |
| SEM | - | 8.19 | 12.20 | 29.92 | 37.169 | 0.034 | 0.035 | 0.084 | 0.030 |
|
P value |
- | 0.027 | 0.0001 | 0.632 | 0.148 | 0.009 | 0.270 | 0.002 | 0.005 |
a, b means in each column, within each factor, bearing the same superscripts are not significantly different. *β-G+MOS= β-Glucan + Mannan oligosaccharides, YCW= Yeast Cell Wall, MR= Mushroom and D= Day.
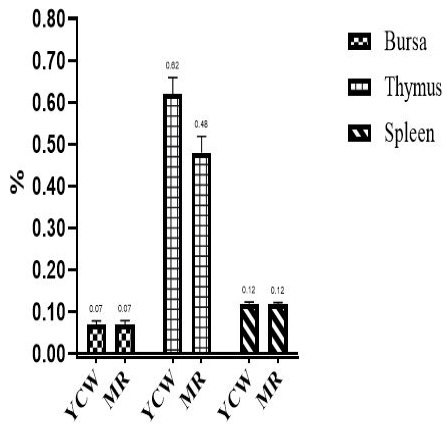
Figure 1: Effect of β-Glucan + MOSs sources on relative lymphoid organs weight. YCW= Yeast cell wall; MR= Mushroom.
Carcass characteristics
Effects of different β-G+MOS sources, levels and their interactions on carcass characteristics are presented in Table 4. There were no significant differences in liver, heart and gizzard% due to either β-G+MOS sources and levels or treatments. Carcass % was significantly (P= 0.05) increased with increasing β-G+MOS level, while no significant differences were observed due to different sources. The group fed 300 ppm β-G+MOS from YCW (T4) recorded the highest carcass% +4.52% over the control.
Results show that dietary β-G+MOS addition to broiler diets significantly improved the growth performance regardless either source or level compared to control one. These results might be due to the beneficial effect of β-G+MOS via several ways. 1-Enhancing the beneficial bacteria populations in broilers gut, as lactobacilli and yeast which enhance production of short chain fatty acids produced via fermentation, which in turn sustain gut health (Spring et al., 2000; Kocher et al., 2005; Yang et al., 2007; 2008b; Swi et al., 2010), 2-Inhibiting the adhesion of pathogenic bacteria and remove them from the gut of broiler such as Salmonella and E.coli and enhance the immune status (Spring et al., 2000, 2015; Ferket, 2004). 3-Developing gut morphology, protecting the broilers from primary infections by increasing goblet cell density which may led to promote performance (Reisinger et al., 2012), 4-Increasing ileal dry matter, crude protein and ether extract digestibility with MOS addition to E. coli challenged as in laying hen diets (Jahanian and Ashnagar, 2015), and 5-Increasing the absorption surface via increasing villi length (Baurhoo et al., 2007). Additionally, no significant differences between sources and levels of β-G + MOS, although chicks fed YCW were showed slight improvement in performance. It might be caused by several reasons; (1) YCW protein content is 15-30% and a small portion of chitin (ERUASYP, 2015). (2) The added amount of YCW was more than MR to cover the graded levels of β-G+MOS being 100, 200 and 300 ppm. (3) Increasing MOS percentage in YCW (20%) vs. MR contains (5%).
Our results were in agreement with Cheraghi et al. (2014) who reported that dietary β-G+MOS improved BWG when broiler chicks fed combination of 24% β-G+ 10% MOS up to 1000 ppm compared to control. Reisinger et al. (2012) concluded that broilers fed 0.1% yeast derivative (0.017% and 0.025% of MOS and β-G, respectively) enhanced LBW, LBWG and FCR compared to control group. Similarly, Tian et al. (2016) reported that supplementation of broiler diets with 200 ppm yeast derived β-G in Clostridium perfringens challenged broiler chicken diets, improved growth parameters by reduce the gut wall damage and increase villi height compared to challenged control group. Baoan et al. (2019) observed an improvement in broiler chickens growth performance when 1000-2000 ppm of yeast β-1, 3/1, 6-glucan was supplemented in their diets compared to control by improving nutrient digestibility due to improve intestinal morphology and increase the counts of intestinal microflora. Cheng et al. (2018) concluded that dietary MOS supplementation at 1000 ppm enhanced growth performance of the broiler chicks. Benites et al. (2008) suggested that birds fed MOS (0.1/0.05/0.05% of starter/ grower/ finisher diet, respectively) significantly increased LBW compared to the control via maintaining the healthy balance of microflora within gut that may improve nutrients efficiency. Similarly, Shendare et al. (2008) concluded that addition of 0.1% MOS significantly improved BWG and FCR of broiler chickens compared to the control group. Zikic et al. (2011) reported that supplementation of MOS (0.1/0.075/0.05% in starter, grower and finisher diet, respectively) led to better broilers BWG than un-supplemented group, with a slight improvement in FCR.
On the other hand, some researchers reported that β-G+MOS in broiler chicken diets didn’t improve their growth performance (Yalcinkaya et al., 2008; Yang et al., 2008a). Chae et al. (2006) reported that no significant differences were observed in BWG, FC and FCR during the starter period. M’Sadeq et al. (2015) reported that supplementation of YCW at (400, 800 and 200 ppm, respectively) during (starter, grower and finisher period, respectively) in broiler diets had no significant effect on broiler performance between 0 to 10 days of age.
Table 4: Effect of β-G+MOS source, level and treatments on carcass characteristics of broiler chicks at 35 days of age.
|
β-G+MOS sources |
β-G+MOS levels (ppm) |
Carcass percent | Liver percent | Heart percent | Gizzard percent | Giblets percent |
| Source | ||||||
| YCW | - | 72.18 | 2.76 | 0.60 | 1.76 | 5.12 |
| MR | - | 71.28 | 2.84 | 0.60 | 1.76 | 5.21 |
| SEM | - | 0.416 | 0.073 | 0.020 | 0.045 | 0.073 |
|
P value |
- | 0.14 | 0.41 | 0.95 | 0.98 | 0.41 |
| Level | ||||||
| - | 100 |
70.68b |
2.66 | 0.57 | 1.83 | 5.07 |
| - | 200 |
71.98ab |
2.88 | 0.62 | 1.73 | 5.25 |
| - | 300 |
72.54a |
2.85 | 0.60 | 1.72 | 5.13 |
| SEM | - | 0.510 | 0.090 | 0.025 | 0.055 | 0.089 |
|
P value |
- | 0.05 | 0.19 | 0.36 | 0.34 | 0.38 |
| Treatment | ||||||
| Control T1 | - |
70.69bc |
2.66 | 0.56 | 1.78 | 5.02 |
| YCW T2 | 100 |
70.01c |
2.54 | 0.57 | 1.77 | 4.89 |
| YCW T3 | 200 |
72.65ab |
2.99 | 0.59 | 1.76 | 5.35 |
| YCW T4 | 300 |
73.89a |
2.74 | 0.63 | 1.74 | 5.13 |
| MR T5 | 100 |
71.35bc |
2.78 | 0.52 | 1.88 | 5.24 |
| MR T6 | 200 |
71.32bc |
2.78 | 0.66 | 1.70 | 5.15 |
| MR T7 | 300 |
71.19bc |
2.96 | 0.56 | 1.70 | 5.24 |
| SEM | - | 0.752 | 0.120 | 0.035 | 0.084 | 0.125 |
|
P value |
- | 0.03 | 0.16 | 0.42 | 0.75 | 0.23 |
a, b means in each column, within each factor, bearing the same superscripts are not significantly different. *β-G+MOS= β-Glucan + Mannan oligosaccharides, YCW= Yeast Cell Wall and MR= Mushroom
Table 5: Effect of β-G+MOS source, level and treatments on relative lymphoid organs weight and plasma protein fractions.
|
β-G+MOS sources |
β-G+MOS levels (ppm) |
Relative lymphoid organs | Plasma protein fractions | |||||
| Bursa percent | Thymus percent | Spleen percent | Total protein (g/dl) | Albumin (g/dl) | Globulin (G) (g/dl) | A/G ratio | ||
| Control T1 | - | 0.10 | 0.67 | 0.11 |
4.93b |
2.50b |
2.43b |
1.03ab |
| YCW T2 | 100 | 0.07 | 0.59 | 0.11 |
4.88b |
2.20b |
2.68b |
0.81bc |
| YCW T3 | 200 | 0.07 | 0.68 | 0.11 |
5.50ab |
3.07a |
2.43b |
1.29a |
| YCW T4 | 300 | 0.05 | 0.60 | 0.12 |
5.02b |
2.37b |
2.64b |
0.90bc |
| MR T5 | 100 | 0.06 | 0.53 | 0.12 |
6.05a |
2.56b |
3.49a |
0.80bc |
| MR T6 | 200 | 0.06 | 0.46 | 0.11 |
5.68ab |
2.40b |
3.51a |
0.65c |
| MR T7 | 300 | 0.07 | 0.46 | 0.12 |
4.96b |
2.16b |
2.55b |
0.94bc |
| SEM | - | 0.022 | 0.068 | 0.007 | 0.242 | 0.152 | 0.254 | 0.104 |
|
P value |
- | 0.79 | 0.16 | 0.89 | 0.013 | 0.008 | 0.014 | 0.010 |
a, b means in each column, within each factor, bearing the same superscripts are not significantly different. * β-G+MOS= β-Glucan + Mannan oligosaccharides, YCW= Yeast Cell Wall and MR= Mushroom.
The results indicate YCW source gave significantly higher thymus gland percent than those of MR source, which had important role in immune response by T-cells secretion. Awaad et al. (2011) concluded that the addition of yeast cell wall containing 25% β-G+24% MOS at 2000 ppm to chicken’s diets can enhance immune response against ochratoxicosis beside growth performance. Rathgeber et al. (2008) reported that yeast derived β-G had a positive effect in promoting growth as antibiotic in broiler diets, explaining that β-G may replace antibiotics in stimulating the broilers immune system. Chae et al. (2006) concluded that above 0.2% dietary yeast β-G improved growth performance and immunity in broilers. Matur et al. (2011) found that 0.1% of YCW derivatives (26% β-G+15% MOS) improved the innate immune system in aflatoxin-challenge broiler breeders. Zhang et al. (2008) suggested that 50 ppm of β-G in the diet may improve broilers performance and humoral immune response. It was observed that MOS can activate gut maturation, nutrient absorption and improve growth performance (Safari et al., 2014). Zakeri and Kashefi (2011) concluded that addition of 0.1% MOS significantly enhanced immune response. Shao et al. (2013) reported that 100 ppm of YCW β-glucan to the diet of broilers infected with Salmonella improved gut health and immunity by increasing villus height, villus height/crypt depth ratio, goblet cell count, and IgA expression cells and content in the jejunum. It was reported that MOS and β-G could improve immune response and prevent pathogenic bacteria adhesion within gut (Volman et al., 2008). Ozpinar et al. (2010) reported that the plasma IgG level was significantly increased when birds fed 1500 ppm MOS compared to control group.
The results indicate that no significant differences were observed between treatments for relative lymphoid organs (bursa, thymus and spleen). The results are in agreement with Cengiz et al. (2012) who reported that dietary supplementation of 1000 ppm β-G+MOS for broiler chicks did not affect the relative weight of spleen. Results are not in agreement with that observed by (Guo et al., 2003; Zhang et al., 2008; Morales-López et al., 2009) who reported that yeast β-glucan is responsible for increasing broilers lymphoid organs relative weight. Sedaghi et al. (2013) reported that supplementation of 1000 ppm MOS+β-G to Salmonella enteritidis challenged chicks diets increased the spleen percent. Awaad et al. (2011) recorded that dietary supplementation of 2000 ppm MOS+β-G increased the bursal percent in Ochratoxicated broiler chickens. Usama et al. (2018) concluded that dietary 4000 ppm β-G+MOS increased broilers lymphoid organs percent that may be attributed to prevent pathogenic bacteria colonization and development of microflora within gut thereby increasing the nutrients absorption and utilization. Chand et al. (2019) reported that MOS up to 100 g/kg of feed can be used to increase weight of lymphoid organs. Also, Teo and Tan (2007) reported that MOS increased weight of lymphoid organs which might be due to increasing gut health, goblet cells count and improving beneficial bacteria population and decreasing of pathogenic bacteria counts within gut and therefore better health status of broiler chicks.
Results indicate significant improvements in carcass percent by 4.5% compared to control, while no significant differences were observed between treatments for giblets (liver, heart, and gizzard) percent. Cengiz et al. (2012) reported that dietary supplementation of 1000 ppm β-G+MOS for broiler chicks did not affect the giblets percent. Also, Yalçin et al. (2014) reported that dietary supplementation with 1000, 2000 and 3000 ppm YCW MOS did not affect the relative weights of broiler chickens giblets. While, Sedaghi (2013) reported that 1000 ppm β-G+MOS increased weight of giblets. Waqas et al. (2019) reported that 600 ppm MOS from YCW in broiler diets improved all carcass traits compared to 200 and 400 ppm levels. In the same line, Alzueta et al. (2010) and Fernandes et al. (2014) reported that the improvement in carcass characteristics might be due to the MOS from YCW content of many beneficial nutritive factors such as protein, B complex and minerals. Protein was needed for muscle development, B complex and minerals involved in gluconeogenesis which improved carcass parameters (Combs, 2008). The fact that MOS prevent adhesion of pathogenic bacteria in the gut may have reduced competition for nutrients between pathogens and chicks, thus using more nutrients for building tissues and growth. On the other side, Syed et al. (2020) observed that no significant improvements of MOS prebiotic on carcass characteristics when added to broiler chicken diets at (0, 500, 1000 and 1500 ppm) for 42 days of age. Blair et al. (2004) and Konca et al. (2009) reported that carcass percentage was not affected by dietary supplementing of MOS in turkey chicks.
Concerning blood plasma parameters, there are significant increases in the total protein, albumin and globulin in β-G+MOS treated chicks. This may be a result of stimulating the activity of digestive enzymes, increasing nutrient digestibility, increasing the absorption rate as a result of increasing intestinal villus length and providing plasma total protein and albumin synthesis with more available amino acids. Oni et al. (2020) demonstrated that serum total protein, albumin and globulin were significantly higher for MOS (500 ppm) supplemented chickens than those for the control group. While, Zhang et al. (2012); Putri et al. (2017) did not observe any significant improvements in blood plasma profiles.
Conclusions and Recommendations
Supplementing broiler diets with β-G+MOS at 100-200 ppm regardless the source had positive effects on broilers growth performance and plasma protein fractions comparing to control group. Neither lymphoid organs nor giblets percent was affected by β-G+MOS addition. Generally, no significant differences were observed between β-G+MOS sources and levels with slight improvement for YCW source than MR source.
Novelty Statement
Our results showed that edible mushrooms and yeast cell wall are natural sources of β-Glucan and MOS and may be used as alternatives to antibiotic growth promoters to enhance broilers performance.
Author’s Contribution
All authors are contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
References