The Journal of Advances in Parasitology
Research Article
The Journal of Advances in Parasitology 1 (2): 24 – 26Prevalence of Haemoproteus Sp in Domestic Pigeon at Chittagong and Khulna District in Bangladesh
Muhammad Shafiqul Islam1*1*, Muhammad Abdul Alim1, Shubhagata Das1, Kazal Krishna Ghosh2, Shahnaj Pervin1 Afroza Lipi1 AMAM Zonaed Siddiki1 Muhammad Masuduzzaman1 Shahnaj Pervin1 Muhammad Alamgir Hossain1
- Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4225
- Department of Microbiology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4225
*Corresponding author: [email protected]
ARTICLE CITATION:
Islam MS, Alim MA, Das S, Ghosh KK, Pervin S, Lipi A, Siddiki AZ , Masuduzzaman M, Hossain MA (2014). Prevalence of haemoproteus sp in domestic pigeon at Chittagong and Khulna district in Bangladesh. J. Adv. Parasitol. 1 (2): 24 – 26.
Received: 2014–02–04, Revised: 2014–04–14, Accepted: 2014–04–23
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.jap/2014/1.2.24.26)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
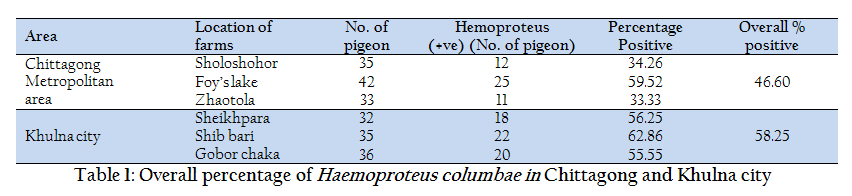
A study was carried out to determine the prevalence of Haemoproteus sp in domestic pigeon at Chittagong and Khulna district in Bangladesh. A total of 213 blood samples were collected from six different locations in Chittagong (Foy’s lake, Bibrhat, Zhaotola) and Khulna district (Banorgati, Sheikhpara, Goborchaka). Blood smears were stained with geimsa and examined under microscope using immersion oil. Mature and immature stages of Haemoproteus gametocytes were found in 43.63% and 58.25% in Chittagong and Khulna respectively. The prevalence was almost similar in the areas of Khulna district (55.55 – 62.86%), whereas, a fluctuation observed in Chittagong district from 33.33 to 59.52%. Finally, we recommended further extensive studies by molecular characterization which will help to make a parasitic lineage.
INTRODUCTION
Pigeons are used as pets, cultural and religious symbols. They also have value as a source of food, hobby and experimental purposes (Sari et al., 2008). Pigeons are affected with several health problems, whereas parasite infections play a major role. Parasitic infection can lead to retarded growth, low egg production and susceptibility to other infections in birds (Dranzoa, 1999). However, parasitic protozoa Haemoproteus sp are widely distributed in tropical and subtropical regions (Adriano and Cordeiro, 2001). In a taxonomic review of the haemoproteids parasites of columbids, only H. columbae and H. sacharoyi are valid species (Bennett and Peirce, 1990). Among which H. columbae infects most commonly in pigeon and doves and are transmitted by blood–sucking louse fly Pseudolynchia canariensis, which is a proven vector of H. columbae (Bennett et al., 1993). Based on the morphological features of the blood stages over 140 species of avian Haemoproteus have been described (Bishop and Bennett, 1992). Some of which are responsible for severe pathology in birds and in pigeons only cause’s disease in stressed condition (Zinkl, 1986). Many recent studies have been recorded on avian blood parasites in different areas of the world (Bensch et al., 2000; Ricklefs et al., 2005). Based on microscopic examination the prevalence of Haemoproteus sp was recorded 20% in Mymensingh district in Bangladesh (Dey et al., 2010). However, there is no study regarding Haemoproteus sp infection in Chittagong and Khulna region, that’s why, the present study was performed to detect the Haeomoproteus sp and address the pigeon farmers about its amplitude.
MATERIALS AND METHODS
Study Area
The study was carried out from April 2012 to may 2013 involving randomly selected 110 and 103 domestic pigeons (Columba livia) from different location at Chittagong (Bibirhat, Foy’s lake and Zhaotola) and Khulna (Sheikhpara, Shib bari and Gobor chaka) respectively.
Sample Collection and Examination
Blood samples for the preparation of blood smears were obtained from a brachial vein and the pigeon then tagged and released. The air–dried blood smears were subsequently fixed in absolute methanol 3–5 minutes and later stained with Giemsa’s with phosphate buffer (pH 7.4) in the ratio of 1:7 for 30 minutes. All dried smears were examined under light microscopy for the detection of blood protozoa and identified according to Soulsby (1982).
RESULTS AND DISCUSSIONS
All samples were microscopically examined and gametocytes of Haemoproteus sp.were seen within red blood cells. Diagnostic characters included the elongated, crescent–shaped forms of the gametocyte, its nucleus size and position in the cell (Picture 1–A). Mature macrogametocytes were circumnuclear and lightly staining blue with Giemsa; refractile granules were found in various sizes, generally moderately large and ovoid, occasionally smaller (Picture 1–C). Whereas, microgametocyte stained pale pink including pigments granule occupied in polar position (Picture 1–B). The characters of macro and micro gametocytes of the present study were in line with Youssefi and Rahimi (2011). Furthermore, Mushi et al. (1999) found H. columbae gamets (immature and mature gametocytes) in blood samples of 75% pigeons through microscopic examination.
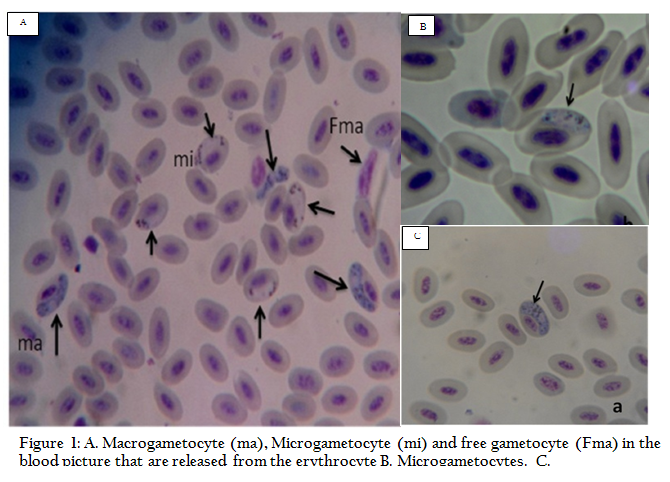
Figure 1: A. Macrogametocyte (ma), Microgametocyte (mi) and free gametocyte (Fma) in the blood picture that are released from the erythrocyte; B. Microgametocytes; C. Macrogametocytes.
In the current study, out of 110 and 103 samples, 48 (46.60%) and 60 (58.25%) were found positive in Chittagong and Khulna district respectively (Table 1). In contrary, studies regarding Haemoproteus infection conducted at Mymensingh district in Bangladesh (Dey et al., 2010), Bursa region (Senlik, 2005) and Madagascar (Raharimanga et al., 2002) revealed prevalence rate ranges from 19–21%. However, the present study was corresponding with Dranzoa et al., (1999); Orajaka and Nweze (1991) who reported that 76.5% and 37.5% pigeons are positive for Haemoproteus columbae respectively in Uganda and Nigeria. Infection load was highest in Khulna City (55.55–62.86%) in comparison with Chittagong district and the infection was lowered in two areas of Chittagong district (Zhaotola 33.33% and Sholoshohor 34.26%) in Columba livia. These differences might be due to climatic condition, population density and husbandry practices of pigeon. In the present study, samples from healthy pigeons exhibited the high infection load of Haemoproteus sp. This high prevalence could be attributed to the presence of blood sucking vector flies Pseudolynchia canariensis (Bennett et al., 1993). Dey et al., (2010) found utmost association of Pseudolynchia canariensis (90%) with the developmental stage of Haemoproteus spp in Mymensing, Bangladesh.
CCONCLUSION
In conclusion, our study indicated that more studies are required owing to different prevalence rates in different parts of Bangladesh. Haemoproteus sp should therefore be considered a economic threat for pigeon farmers’ although there is no published data available in our country.
REFERENCES
Adriano AE, Cordeiro SN (2001). Prevalence and Intensity of Haemoproteus columbae In three Species of Wild Doves from Brazil.Mem.Inst .Oswaldo. Cruz, Rio de Janeiro, Vol. 96 (2): 175–178.
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H, Pinheiro RT (2000). Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. Lond. B. 267: 1583–1589.
http://dx.doi.org/10.1098/rspb.2000.1181
PMid:11007335 PMCid:PMC1690711
Bennett GF, Peirce MA (1990). The haemoproteid parasites of the pigeons and doves (family Columbidae).J. Nat. Hist. 24: 311–325. .
http://dx.doi.org/10.1080/00222939000770231
Bennett GF, Pierce MA, Ashford RW (1993). Avian haematozoa: Mortality and pathogenecity. J. Natur. Histo. 27: 993–1001.
http://dx.doi.org/10.1080/00222939300770621
Bishop MA, Bennett GF (1992). Host–parasite catalogue of the avian haematozoa: Supplement 1 and Bibliography of the avian blood–inhabiting haematozoa: Supplement 2. Occasional Papers in Biology. Memorial University Newfoundland, 15, 1–244.
Dey AR, Begum N, Paul SC, Noor M, Islam K (2010). Prevalence and pathology of blood protozoa in pigeons reaered at Mymensingh district, Bangladesh. Int. J. Bio. Res. 2 (12): 25–29.
Dranzoa C, Ocaido M, Katete P (1999). The ecto– gastro–intestinal and haemo–parasites of live pigeons (Columba livia) in Kampala, Uganda. Avi. Pathol. 28(2): 119–124. .
http://dx.doi.org/10.1080/03079459994830
Mushi EZ, Binta MG, Chabo RG, Mathaio M, Ndebele RT (1999). Haemoproteus Columbae in domestic pigeons in Sebele, Gaborone, Botswana. Ond. J. Vet. Res. 66: 29–32.
Orajaka LJ, Nweze LC (1991). Prevalence of blood protozoan parasites of avian species in Nsukka area of Anambra State, Nigeria. Beitr. Trop. Landwirtsch. Veterinarmed. 29(1): 91–95. .
PMid:1930107
Raharimanga V, Soula F, Raherilalao MJ, Goodman SM, Sadonès H, Tall A, Randrianarivelojosia M, Raharimalala L, Duchemin JB, Ariey F, Robert V (2002). Hemoparasites in wild birds in Madagascar. Archi. Insti. Paste. Madaga. 68(1–2): 90–94. .
PMid:12643101
Ricklefs RE, Fallon SM, Latta SC, Swanson BL, Bermingham E (2005). Migrants and their parasites: a bridge between two worlds, In: Birds of Two Worlds: The Ecology and Evolution of Migrants, The Johns Hopkins University Press, Baltimore, 210–221.
Sari B, Karatepe B, Karatepe M, Kara M (2008). Parasites of domestic pigeon (Columba livia domestica) and wild (Columba livia livia) pigeons in Niğde, Turkey. Bull. Vet. Inst. Pulawy. 52: 551–554.
Senlik B, Gulegen E, Akyol V (2005). Prevalence and intensity of Haemoproteus columbae in domestic pigeons. Ind. Vet. J. 82(9): 998–999.
Soulsby EJL (1982). Arthropods and Protozoa of Domesticated Animals (7th Ed), ELBS and Bailliere Tindall, London, 700–706.
Youssefi MR, Rahimi MT (2011). Haemoproteus Columbae in Columba livia domestica of Three Areas in Iran in 2010. Glob. Vet. 7 (6): 593–595.
Zinkl JG (1986). Avian Haematology, in Schalm's Veterinary Haematology (4th Ed), Philadelphia: Lea & Febiger, 256–273.