Advances in Animal and Veterinary Sciences
Research Article
Mechanisms of Hepato-Renal Protective Activity of Ocimum basilicum Leaf Extract against Paracetamol Toxicity in Rat Model
Ahmed M. Soliman1*, Hanan A. Rizk3, Mostafa A. Shalaby1, Ashraf A. Elkomy2
1Department of pharmacology, Faculty of Veterinary Medicine, Cairo University, Egypt; 2Department of pharmacology, Faculty of Veterinary Medicine, Benha University, Egypt; 3Department of pharmacology and toxicology, National Organization for Drug Control and Research (NODCAR), Egypt.
Abstract | The present study aimed to investigate the hepato-renal protective effect of Ocimum basilicum (O. basilicum) leaf extract against the hepato-renal toxicity induced by paracetamol in rats. Twenty four Wistar male rats were divided into four groups each group contains 6 rats as follows: Group I rats received 2 ml distilled water for 30 days and served as a vehicle control. Rats in the group II were given single oral dose of paracetamol (500 mg/kg), 1 h after last distilled water administration and kept as paracetamol intoxicated control group. Groups III and IV received the ethanolic extract of O. basilicum at 200 and 400 mg/kg bwt, respectively, once daily for 30 consecutive days followed by a single oral dose of paracetamol (500 mg/kg), 1 h after the last O. basilicum extract. The degree of hepatoprotection was measured using liver enzymes (aspartate aminotransferase (AST), alanine aminotransferase (ALT) and bilirubin, albumin, and lipid profile. The degree of renal protection was measured using creatinine, urea, total protein and glucose. Histopathological examinations of liver and kidney were also done. The significantly altered liver and kidney functions by paracetamol toxicity were restored to nearly normal values by administration of O. basilicum extract. Change in biochemical parameters in groups received paracetamol alone was normalized in groups given paracetamol and O. basilicum extract. Histopathological lesions induced by paracetamol in liver and kidney was also markedly decreased by its co-administration with O. basilicum extract. The findings indicated that O. basilicum extract has a hepato-renal protective activity against paracetamol induced toxicity in rats. This protection may be attributed to good antioxidant activity of O. basilicum. Therefore intake of O. basilicum extract may reduce hepato-renal toxicity induced by paracetamol.
Keywords | Ocimum basilicum, Paracetamol, Hepato-renal toxicity, Antioxidant
Received | January 03, 2020; Accepted | March 12, 2020; Published | March 20, 2020
*Correspondence | Ahmed M. Soliman, Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; Email: [email protected]; [email protected]
Citation | Soliman AM, Rizk MA, Shalaby MA, Elkomy AA (2020). Mechanisms of hepato-renal protective activity of Ocimum basilicum leaf extract against paracetamol toxicity in rat model. Adv. Anim. Vet. Sci. 8(4): 385-391.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.385.391
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Soliman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The use of medicinal plants in folk medicine is an age-long practice in various parts of the globe for both preventive and curative purposes. Todays, it is estimated that about 80% of the world population relies on botanical preparations as medicine to meet their healthcare needs (Ogbera et al., 2010).
Liver is the largest organ in the body and plays a significant role in protecting various biological function and help in detoxification and excretion of various endogenous and exogenous compounds (Mohamed et al., 2010). The liver is often the target organ for many chemically induced injuries. Many oxidative reactions produce reactive metabolites that can induce oxidative stress and consequently liver damage or injury. The types of injury to the liver depend on the type of toxic agent, the severity of intoxication, and the type of exposure, whether acute or chronic toxicity (Comfort et al., 2013).
Kidneys has an important role in removing wastes like creatinine and urea, regulating the balance of electrolytes and controlling the body’s fluid balance (Walter, 2004). For the kidneys to carry out their normal functions they have to be in good condition both functionally and structurally (Thomas, 2005).
Paracetamol (PCM); is a widely used analgesic medication in many countries. An overdose of paracetamol is a frequent reason for liver and renal toxicity and possible death (Zhao et al., 1998). PRM is metabolized in the liver by cytochrome P450 to N-acetyl-p-benzoquinone imine (NAPQI). NAPQI reacts with glutathione (GSH), therefore overdoses of paracetamol may result in a depletion of hepatocellular GSH (Hinson et al., 2004). GSH exhaustion caused NAPQI to binds with cellular proteins leading to mitochondrial dysfunction, oxidative stress, lipid peroxidation, DNA fragmentation, massive hepatocytes necrosis, liver damage and death (Larson, 2007).
Ocimum basilicum (Basil) is an annual herb of the Lamiaceae family, which is widely cultivated in different regions of the world. O. basilicum was found to have numerous pharmacological activities. Basil leaves extracts have potent antioxidant, anti-aging, anticancer, antiviral, and antimicrobial properties (Akujobi et al., 2004; Manosroi et al., 2006; Almeida et al., 2007). It also possesses good antioxidant as well as potential anti-stress in experimental animals (Sethi et al., 2004). Leaves and flowering parts of O. basilicum are traditionally used as antispasmodic, aromatic, carminative, digestive, galactogogue, stomachic and tonic agents (Adiguzell et al., 2005). O. basilicum is known to have numerous pharmacological activities. Basil leave extracts have potent antioxidant, anti-aging, anticancer, antiviral, and antimicrobial properties (Dasguptazz et al., 2004; Bozin et al., 2006; Noor et al., 2019; Eftekhar et al., 2019). The volatile oils of O. basilicum are estragol, linalool, eugenol, methyl chavicol and small quantities of methyl cinnamate, cineole, apigenin, luteolin, orientin, vicenin, and other terpenes (Samudralwar and Garg, 1996). Consumption of O. basilicum or basil oil has been associated with a reduction of total cholesterol, low-density lipoprotein and triglycerides (Hicham et al., 2009)
Hence, the goal of the present research work was to examine if the hepato-renal toxicity induced by paracetamol can be ameliorated by the use of O. basilicum extract or otherwise.
MATERIALS AND METHODS
Medicinal plant
The fresh leaves of O. basilicum, were were collected from a garden of Faculty of Agriculture, Cairo University, Egypt. Leaves were rinsed with clean water to remove any foreign matter, air dried and grinded into fine powder.
Preparation of ethanolic extract
Organic solvent extraction of the O. basilicum leaves was carried out using 95% ethanol which is considered as very effective solvent in extracting the active ingredients of the plant according to method described by Effraim et al. (2000). This was done using Soxhlet apparatus, fifty g of plant leaves powder were soaked in 500 ml of 95% ethanol inside the flask. The extraction was carried out for 24 hours by heating temperature that kept the solvent at 50-60 °C until a clear and colorless solvent appeared in the extracting unit. After that, the extract was dried by using an electric oven at temperature 40-45 °C until semi-solid extract was obtained. The dried extract was placed in an incubator at 38-40 °C for complete dryness of the sample. The final extract was kept frozen at –20 °C until use.
Experimental rats
Male Wistar rats (185-210 g) were obtained from the Animal House, Faculty of Veterinary Medicine, Cairo University, Egypt. They were fed on a standard rat pellet and tap water was provided ad libitum. Rats were kept in plastic cages at room temperature 22-25°C. Experimental rats were acclimatized to the environment for two week prior to experimental use. This experiment was carried out according to the guidelines of the Institutional Animal Care and Use Committee, Cairo University, Approval Protocol No.: Cairo University-II-F-59-15 dated December/2018. (Protocol number 2211201809 / 2018).
Experimental protocol
Twenty four male Wistar rats were distributed into four groups of six rats each. Group I rats received 2 ml of distilled water for 30 days and served as a vehicle control. Rats in the group II served as paracetamol intoxicated control and were administered a single oral dose of paracetamol (500 mg/kg) (Zhang et al., 2013), 1 h after last dose of distilled water administration. Groups III and IV received ethanolic extract of O. basilicum in dose of 200 and 400 mg/kg, respectively, once daily for 30 consecutive days followed by a single oral administration of paracetamol (500 mg/kg), 1 h after the last O. basilicum extract administration.
Blood sampling
After 24 h of paracetamol administration, rats were anesthetized by intraperioneal dose (50mg/kg) of pentobarbital sodium (Nesdonal®). Blood samples (2 ml) from each rat was collected by puncturing tail vein with fine needle in sterilized dry centrifuge tube and left to clot for 30 min at room temperature. The clear sera obtained after centrifugation (3000 rpm for 15 min) were stored at 4 ºC till used and used for further biochemical estimation which was performed by using ready-made kits from Diamond Diagnostics Company (Egypt). Rats were then euthanized by large dose (60 mg/kg) of pentobarbital sodium and livers and kidneys were dissected out for histopathological examination.
Statistical analysis
Data were expressed as (mean ± SE) and analyzed using SPSS (Statistical Package for the Social Sciences, version 16.0, Illinois, Chicago, USA) and differences between the means were examined by Duncan’s multiple range test.
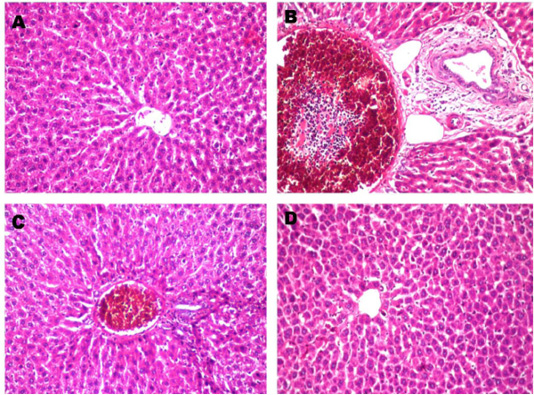
Figure 1: Histopathological changes in liver after treated with paracetamol and/or Ocimum basilicum. A: Normal liver section from control rat shows no histopathological alteration and the normal histological structure of the central vein and surrounding hepatocytes in the parenchyma (X10). B: Paracetamol treated rats showed sever congestion and dilatation in the portal vein associated with inflammatory cells infiltration and oedema in the periductal tissue surrounding the cystic dilated bile ducts (X20). C: Paracetamol + Ocimum basilicum (200 mg/kg bwt) treated rats, showed congestion in the portal vein associated with few inflammatory cells infiltration surrounding the bile ducts (X10) and D: Paracetamol + Ocimum basilicum (400 mg/kg bwt) treated rats, there was no histopathological alteration were recorded (H and E).
RESULTS
Oral administration of paracetamol at a dose of 500 mg/kg caused a significant increase in parameters of liver function (ALT, AST, bilirubin, total cholesterol, triglycerides, LDL-c) and a significant decrease in albumin and HDL-c in comparison with control values. These biochemical parameters are recorded in Table 1. Oral administration of paracetamol at a dose of 500 mg/kg caused a significant increase in kidney function parameters (creatinine, urea, Glucose, sodium and potassium) and significant decrease in total protein in comparison with control values, these biochemical parameters are recorded in Table 2. In O. basilicum extract treated groups these biochemical parameters were returned towards the normal values. Liver and renal oxidative stress marker and antioxidant parameters in control and different treated groups are mentioned in Tables 3 and 4, respectively.
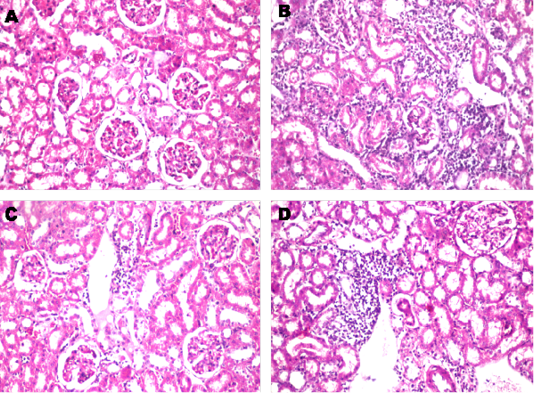
Figure 2: Histopathological changes in renal cortical tubules after treated with paracetamol and/or Ocimum basilicum. A: Kidney section from control rat shows the normal histological structure of the glomeruli and tubules at the cortex (X10). B: Paracetamol treated rat shows, Massive inflammatory cells infiltration was detected in between the degenerated renal tubules at the cortex (X10). C Paracetamol + Ocimum basilicum (200 mg/kg bwt) treated rats, showed focal inflammatory cells infiltration was detected in the perivascular area surrounding the cortical blood vessels (X10) and D: Paracetamol + Ocimum basilicum (400 mg/kg bwt) treated rats, focal inflammatory cells infiltration was detected in between the tubules at the cortex (H and E).
DISCUSSION
Paracetamol induced hepatic injury is considered as one of the most commonly used model for hepatoprotectivity drug screening through estimation of serum cytoplasmic enzymes; aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum albumin (SA), total bilirubin (TB), blood urea (BU) and serum creatinine (SC) activities is a useful as quantitative markers of the extent, type of hepatocellular and renal affection by paracetamol (Dash Deepak et al., 2007). In the current study, the significant elevation of these biochemical parameters in the rats treated with paracetamol, indicate the deterioration of the hepatic functions due to the toxic effects of the drug and consequently, they have been attributed to the damage of structural integrity of the liver, because these enzymes released into the circulation after autolytic breakdown or cellular necrosis (Zhang et al., 2009).
Paracetamol treated group of rats showed marked increase in serum creatinine and urea values as compared to the values of serum creatinine and urea in rats of healthy control group. Increased levels of serum creatinine and urea have been considered as index of assessing nephrotoxicity (Anwar et al., 1999; Ali et al., 2001). The elevated values in
Table 1: Effect of oral administration of ethanolic extract of Ocimum basilicum (200 and 400 mg/kg b.wt. on liver parameters in paracetamol induced hepato-renal toxicity in rats (n=6).
| Parameters | Control | Paracetamol |
Ocimum basilicum extract (200 mg/kg bwt) + paracetamol |
Ocimum basilicum extract (400 mg/kg bwt) + paracetamol |
| AST (U/L) |
113.6±3.56b |
151.33±4.06a |
133.3±10.69ab |
128.63±1.55b |
| ALT (U/L) |
25.60±0.58b |
54.12±4.61a |
38.99±2.43ab |
34.34±2.71b |
| T. bilirubin (mg/dl) |
0.47±0.03c |
0.87±0.03a |
0.67±0.07b |
0.60±0.06bc |
| Albumin (g/dl) |
5.15±0.28a |
3.55±0.14b |
4.15±0.27b |
4.31±0.18b |
| Cholesterol (mg/dl) |
66.33±5.04b |
94.33±6.33a |
82.67±3.17ab |
71.33±4.33b |
| Triglycerides (mg/dl) |
63.33±3.52b |
103±2.88a |
76.01±6.50b |
75.33±5.84b |
| HDL-Chol (mg/dl) |
46.67±0.67a |
29.67±2.84b |
39.33±0.67a |
40.33±4.18a |
| LDL-Chol (mg/dl) |
11±0.27b |
44.07±2.769a |
28.13±2.37ab |
15.93±1.23b |
a, b, c Mean values having different letters in the same row differ significantly P<0.05).
Table 2: Effect of oral administration of ethanolic extract of Ocimum basilicum (200 and 400 mg/kg b.wt. on kidney parameters in paracetamol induced hepato-renal toxicity in rats (n=6).
| Parameters | Control | Paracetamol |
Ocimum basilicum extract (200 mg/kg bwt) + paracetamol |
Ocimum basilicum extract (400 mg/kg bwt)+ paracetamol |
| Creatinine (mg/dl) |
1.13±0.09c |
2.89±0.11a |
1.85±0.04b |
1.93±0.07b |
| Urea (mg/dl) |
57.24±4.14b |
70.81±5.41a |
40.57±2.01c |
26.41±0.55d |
| T. protein (g/dl) |
20.25±1.77a |
15.69±1.62b |
12.81±0.92b |
7.78±0.28c |
| Glucose (mg/dl) |
49.75±2.76b |
108.28±9.32a |
65.93±2.76b |
63.72±3.24b |
a, b, c, d Mean values having different letters in the same row differ significantly (P<0.05).
Table 3: Antioxidant activity of Ocimum basilicum (200 and 400 mg/kg b.wt) in liver tissues (n=6).
| Parameters | Control | Paracetamol |
Ocimum basilicum extract (200 mg/kg bwt) + paracetamol |
Ocimum basilicum extract (400 mg/kg bwt) + paracetamol |
| CAT (U/g.tissue) |
54.76±4.58a |
12.60±0.66c |
26.89±1.45b |
22.13±0.71b |
| SOD (U/g.tissue) |
47.24±2.17a |
19.46±1.72c |
27.88±2.54bc |
33.40±2.54b |
| MDA (U/g.tissue) |
35.27±2.73c |
94.24±4.81a |
67.88±3.36b |
42.58±3.25c |
a, b, c Mean values having different letters in the same row differ significantly (P<0.05).
Table 4: Antioxidant activity of Ocimum basilicum (200 and 400 mg/kg b.wt) in kidney tissues (n=6).
| Parameters | Control | Paracetamol |
Ocimum basilicum extract (200 mg/kg bwt)+ paracetamol |
Ocimum basilicum extract (400 mg/kg bwt) + paracetamol |
| CAT (U/g.tissue) |
73.80±6.29a |
23.80±1.30d |
54.76±4.75b |
40.47±2.38c |
| SOD (U/g.tissue) |
122.15±8.38a |
73.62±2.89c |
100.95±2.88ab |
99.61±4.35ab |
| MDA (U/g.tissue) |
18.22±1.37c |
76.20±6.98a |
66.25±6.32a |
29.90±1.80b |
a, b, c Mean values having different letters in the same row differ significantly (P<0.05).
experimental rats indicate the severity of kidney damage by the Paracetamol. Necrosis of liver and kidney cells observed in the present study might be responsible for elevation of these biomarker enzymes. Values of serum creatinine and urea in groups of rats treated with O. basilicum extract showed significant improvement and serum creatinine and urea values. Previous studies reported the significant decrease in serum creatinine level by treatment with various herbal preparations and extracts which was increased in Paracetamol induced nephrotoxic rats support the findings of present study (Gulnaz et al., 2010; Palani et al., 2010).
This study demonstrated that, Alterations in serum levels of hepatic transaminases (AST and ALT) were used as markers for liver damage and injury. In our study, there was a significant increase in AST and ALT levels in paracetamol administered rats. O. basilicum administration ameliorated both liver and kidney changes confirming the protective role of O. basilicum against hepatic toxicity induced by paracetamol overdose and that is coincided with results of Li et al. (2013). Moreover, it was reported that O. basilicum supplementation improved liver histopathology and showed an improvement in hepatic toxicity.
Lipid peroxidation and antioxidant potency (SOD and catalase) of cells were used to assess the degree of hepatic cell stability and integrity (Evans et al., 2002). Oxidative damage caused by paracetamol overdose was significantly attenuated by O. basilicum administration.
Ocimum basilicum extract produced significant decrease in triglycerides, total cholesterol, LDL-cholesterol and significant increase in HDL-cholesterol concentrations in serum of O. basilicum extract treated groups than recorded in control group. Also, improve the lipid profile after paracetamol toxicity. O. basilicum treatment attenuated serum lipid profile. This may be due to the antihyperlipidemic action of components of O. basilicum leaves. Suanarunsawat et al. (2009) mentioned that the anti-hyperlipidemic activity of O.basilicum may be due to the suppression of liver lipid synthesis. These results were consistent with those obtained by Elkomy et al. (2016) who recorded that, paracetamol overdose caused significant increase in serum total cholesterol, serum triglycerides, low density lipoprotein, total bilirubin with a reduction of albumin and high density lipoprotein cholesterol.
Previous phytochemical screening revealed that O. basilicum is a rich source of polyphenols which have potential therapeutic and health promoting effects (Garcia-Lafuente et al., 2009). Polyphenolic compounds are most abundant natural antioxidants and their radical scavenging capabilities play an important role in preventing many chronic diseases (Garcia-Lafuente et al., 2009; Rathee et al., 2009). The main groups of polyphenols are: flavonoids, phenolic acids, phenolic alcohols, stilbenes, and lignans (D’Archivio et al., 2007). The antioxidant activity of the plant may be due to flavonoids which have shown to possess various biological properties related to antioxidant mechanism (Marzouk, 2009), or the effect may be due to Caffeic acid (CA): which is another content in the leaf of the O. Basilicum which has antioxidant, anti-inflammatory, and cancer chemopreventive activities (Neradil et al., 2003). Other constituent of O. Basilicum is a p-coumaric acid (pCA) which found in high concentration which possessed radical scavenging and antioxidant (Yeh and Yen, 2003). Recent studies reported that the antioxidant activity of Basil (Ocimum basilicum leaves) could be attributed to its bioactive phenolic compounds in leaves and flowers (Prinsi et al., 2020). In addition, other researchers (Noor et al., 2019; Eftekha et al., 2019) reported that ocimum basilicum has high antioxidant activity against oxidative stress. It also has immunomodulatory, antinflammatory, antiapoptotic and cell regeneration effects (Ibrahim et al., 2020).
All above mechanisms may acceleration of liver cells regeneration and reducing the leakage of the above enzymes in to blood. On the other hand ethanolic leaves extract of O. Basilicum caused decreased in the level of cholesterol and this result in agreement with previous studies (Amrani et al., 2006).
Histopathological findings of tissue damage induced by paracetamol in the liver showed sever congestion and dilatation in the portal vein associated with inflammatory cells infiltration and edema in the periductal tissue surrounding the cystic dilated bile ducts and this coincides with Roomi et al. (2008) who found mild and significant lobular focal hepatitis in paracetamol studied group. Regarding renal histological finding, massive inflammatory cells infiltration was detected in between the degenerated renal tubules at the cortex. These results are in agreement with those of Pathan et al. (2013) who reported that kidneys of paracetamol-treated rats, showed loss of renal tubular architecture with rearrangement of renal tubules and glomeruli. Kidney sections showed diffuse degenerative changes in the sections and the renal tubules showed cellular swelling invariably with narrowing of lumen to great extent. Histopathological findings of paracetamol on liver and kidney were also markedly decreased by co-administration of O. basilicum extract.
CONCLUSIONS
Ocimum basilicum leaf extract normalized all the treated biochemical parameters of liver and kidney function induced by paracetamol to nearly to normal levels. It also ameliorated the histopatholocal lesions seen in livers and kidneys. Thus the ethanol extract of Ocimum basilicum minimizes the hepato-renal toxicity induced by paracetamol, thereby suggesting its use as a potent hepatoprotective nephroprotective agent.
ACKNOWLEDGMENT
The authors wish to thank Prof. Dr: Adel Bakeer (Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt) for his comments on histopathological study.
AUTHORS CONTRIBUTION
Mostafa A. Shalaby and Ashraf El-Komy designed and planned this study. Ahmed M. Soliman and Hanan A. Rizk prepared the plant extract and performed the experimental work, samples collection and all laboratory tests. All authors shared manuscript writing, drafted and approved the final manuscript.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
REFERENCES






