Advances in Animal and Veterinary Sciences
Review Article
Advances in Animal and Veterinary Sciences 2 (5): 305 – 311Plant Based Edible Vaccines against Poultry Diseases: a Review
Plantharayil Bharathan Aswathi1*, Subrat Kumar Bhanja1, Ajit Singh Yadav1, Valsala Rekha2, Jeny Kalluvila John2, Devi Gopinath2, Anil Shinde1, Aron Jacob2, Gurupriya Vijayasaraswathy Sadanandan2,
- Central Avian Research Institute, Izatnagar, Bareilly, UP, India
- Indian Veterinary Research Institute, Izatnagar, Bareilly, U.P. India
*Corresponding author: [email protected]
ARTICLE CITATION:
Aswathi PB, Bhanja SK, Yadav AS, Rekha V, John JK, Gopinath D, Sadanandan GV, Shinde A, Jacob A (2014). Plant based edible vaccines against poultry diseases: a review. Adv. Anim. Vet. Sci. 2 (5): 305 – 311.
Received: 2014–05–26, Revised: 2014–06–20, Accepted: 2014–06–21
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.5.305.311
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Plant based edible vaccines are one of the novel branches of vaccinology. Candidate antigen can be expressed on selected plant species through various biotechnological approaches. Stable integration of selected antigen into plant genome can be achieved through vector mediated or biolistic method of transgenesis. Another method is transient expression of candidate antigen using agroinfiltration or infection with modified RNA viruses. These types of vaccines have been developed against poultry diseases also. Immunogenic proteins of avibirnavirus (VP2), avian reo virus (σC), Newcastle disease virus (HN, F), avian coronavirus (S1), avian influenza virus (rHA0), chicken infectious anaemia virus (VP1) and Eimeria tenella (EtMIC2) were expressed in selected plants. Oral immunization with these transgenic plants followed by challenge with infectious organisms exhibited protective efficacy. Edible vaccines against poultry diseases are cost effective, thermostable and devoid of human or animal pathogens and microbial toxins. Other points in credit for edible vaccines include suitability for mass vaccination, ease of administration, storage stability, least stress, needle free delivery, no muscle damage etc. Commercial preparations of plant based edible vaccines are likely become a reality in near future. Before that, problems like standardization of expressed antigen concentration, vaccine composition, vaccine efficacy, safety and stability under field conditions have to be looked into. Plant based vaccines against various poultry diseases may become an alternative to conventional vaccination programmes in coming decades.
INTRODUCTION
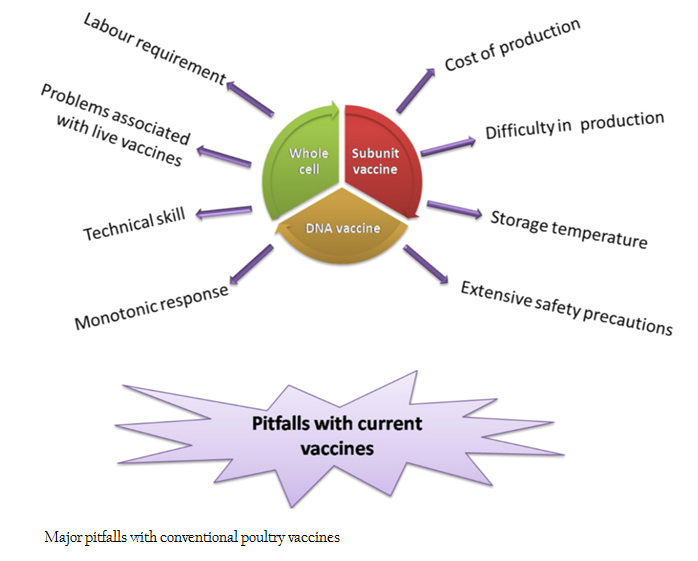
Infectious diseases are a major threat to the poultry industry. Currently, economic losses due to poultry diseases are 10 to 20% of the gross value of production in developed countries and are likely to be higher in developing countries (FAO, 2014). With advancement in the field of vaccinology, various types of vaccines like whole cell vaccines (live attenuated and killed), subunit vaccines, DNA vaccines etc. are available against common poultry diseases. The major pitfalls of existing vaccines are high production cost, difficulty in maintaining cold chain, vaccine safety, problems associated with mass vaccination, manpower and technical skill needed for vaccine administration, complexity in production and purification etc (Nochi et al., 2007; Ferraro et al., 2011; Klein et al., 2013) (Figure 1). With the advent of transgenic technology, development of plant based edible vaccines offers a new prospect to overcome these hurdles. Edible vaccines are reported to provide immune protection equal to or more than that of commercial vaccines (Mason et al., 2002; Walmsley and Arntzen, 2003). Other advantages of plant based vaccines are ease of production, scale up and administration, biological encapsulation of candidate antigen, ability to evoke serum and mucosal response, protection against mucosal pathogens, low production cost, room temperature stability, trouble free storage, devoid of human or animal pathogens, free from pyrogens and microbial toxins, needle free administration etc. (Moffat, 1995; Sala et al., 2003; Streatfield, 2005; Chen and Lai, 2013; Pniewski, 2013; Aboul–Ata et al., 2014).
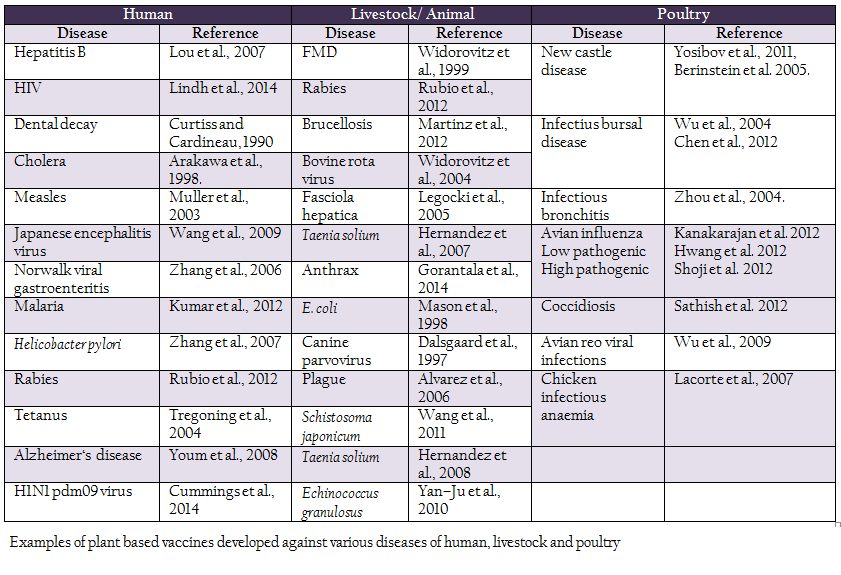
Table 1: Examples of plant based vaccines developed against various diseases of human, livestock and poultry
BACKGROUND
Plant genetic engineering, one of the most important branches of biotechnology, began in early 1970s. As a result of it, dramatic developments in agriculture occurred and research got expanded to explore plant genetic resources for purposes other than nutrition. In 1990, Dr. Charles Arntzen, a plant biotechnologist first put forward the concept of edible vaccines. Major emphasis was given to the production of protective antigens against various human pathogens. First plant expression of a vaccine antigen was done by Dr. Curtiss and Dr. Cardineau in 1990 against Streptococcus mutans which is the causative organism for dental decay in humans. Streptococcus mutans surface protein antigen A (Spa A) was successfully expressed in tobacco plant. Haq et al., (1995) first reported immunization with an edible vaccine (Escherichia coli heat–labile enterotoxin) produced in transgenic plant. Various candidate antigens were expressed in plant tissue against pathogens of human, animals and poultry. Some examples are given in Table 1.
PLANT BASED EDIBLE VACCINE PRODUCTION
Plant based edible vaccines are recombinant protein vaccines, in which selected plant species is used to produce the selected antigen(s) which are capable of inducing protective immunity against particular animal pathogens on their oral delivery in the form of an edible vaccine (Lossl and Waheed, 2011). Plant based vaccines are believed to overcome most difficulties faced by conventional vaccines. They reduce the cost of production and purification, so that economically more affordable vaccines can be manufactured. One of the major problems faced by developing and underdeveloped countries is difficulty in maintaining cold chain from production till vaccine administration. Edible vaccines because of their ability to thrive in room temperature may solve this problem. Oral delivery is more comfortable for poultry and animals as handling stress and pain due to parenteral route administration can be completely eliminated. The transgenic plant part can be used in fresh or dry form. Edible vaccines can elicit both mucosal and serum immune response. Plant based expression system is cost optimized; genetic manipulation is as easy as production and scale up. Moreover, plant based edible vaccines are as safe as traditional vaccines and chance of contamination with other animal pathogens and toxins can also be eliminated (Sala et al., 2003).
COMMONLY USED PLANTS FOR EDIBLE VACCINE PRODUCTION
The most common plant used for expression of protein and vaccine production is tobacco (Nicotiana benthamiana) because of its transforming ability (Dhama et al., 2013). Edible vaccines can also be produced in cereal grains (Oryza sativa, Zea mays), fruits (banana, tomato), leaves (Lettuce, alfalfa, peanut leaves), tubers (Potato, carrot), legume seeds (Cow pea, soyabean) etc. For raw consumption, plant should be palatable. Cooking may destroy the antigenic potential of transgenic plant. To overcome this difficulty, transgenic plants which can be subjected to high temperature cooking without affecting antigenicity are also proposed (Eg. cooked genetically modified corn snack that can harbour E. coli heat labile enterotoxin). For veterinary
REFERENCES
use, different fodder crops and cereal grains that are a part of normal feed formulation can be used for vaccine production which enables easy administration with least chance for rejection. The most effective way for preservation, standardization and administration of edible vaccine is through making dry powder from these antigen containing transgenic plants. In Arntzen’s laboratory, expressed protein stability of transgenic tomato powder was found to be satisfactory for a period up to one year (Sala et al., 2003). The main points to be kept in mind before selecting a plant for expression of candidate antigen are: plant should be hardy, it should be palatable and well accepted as a part of diet, it should be indigenous in nature and easily available and transformable (Dhama et al., 2013).
BIOTECHNOLOGICAL APPROACH
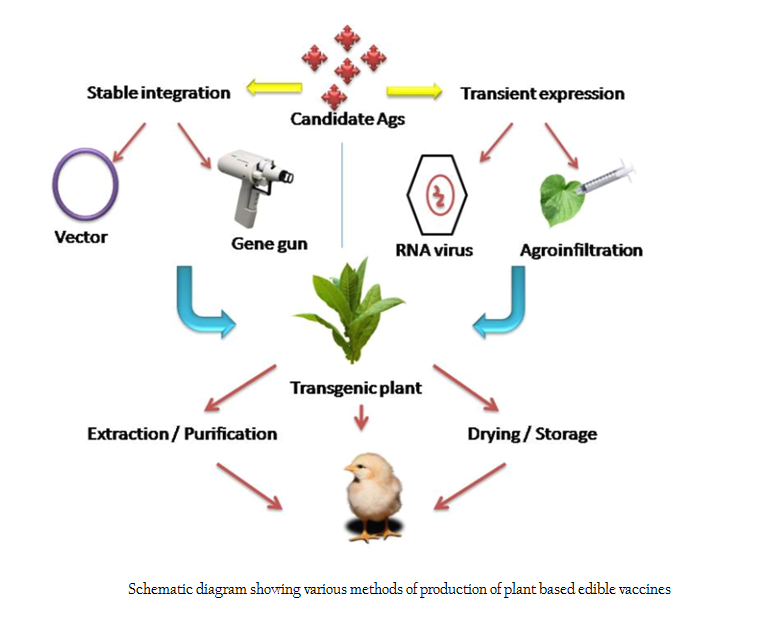
Antigen expression in plants can be achieved through stable integration of candidate antigen into plant cell or using transient expression systems (Figure 2). For stable integration, vector mediated carrier system is followed. Natural plant pathogens like Agrobacterium tumefaciens is used for stable integration of antigenic DNA into plant nucleus. This method is benefited by the inherent capability of plant pathogens to infect and transfer its virulent genes to the nucleus of host cell (Jacob et al., 2013). The main advantages of this method are ease of production, cost effectiveness, ability to introduce large DNA segments with higher efficiencies into the plant genome etc (Gelvin, 2003; Kohli et al., 1998). Agrobacterium mediated gene integration occurs at random chromosomal sites. Gelvin (2003) reported that the newly introduced DNA inside the nucleus will randomly get integrated into host cell genome by non–homologous recombination at the same locus or different loci resulting in formation of stable transgenic plants. An alternative method is integration of candidate gene into plant circular chloroplast DNA (cpDNA) through microprojectile bombardment method (biolistic method) or ‘particle guns’ which results in site specific integration (Daniell et al., 2001; Daniell et al., 2002). In this method, selected DNA sequences are precipitated into metal micro particles and bombarded against the target tissue at an accelerated speed so that microparticles penetrate the cell wall and release exogenous DNA inside the cell and get integrated into the host cell genome (Taylor and Fauquet, 2002.). Candidate antigen integration into cpDNA has several benefits – the cpDNA molecule of important plants has been completely sequenced, presence of more than 10,000 copies of cpDNA per cell enables expression of foreign genes to extremely high levels, transgene integration occurs exclusively by homologous recombination, plastid genetic system lacks gene silencing and other epigenetic mechanisms that impede with stable transgene expression, maternal mode of plastid inheritance in the majority of angiosperm species decreases the chance of transgene transmission through pollen and many plastid genes are arranged in operon offering the possibility to stack transgenes by arranging them in artificial operons (Bock, 2014; ). An alternate polyethylene glycol (PEG) mediated protoplast transformation method is occasionally used (Maliga and Bock, 2011). Though PEG–mediated plastid transformation is technically demanding, laborious and also more time–consuming than biolistics, it has the advantage that the method is not protected by patents. Transient expression of candidate gene can be mediated by positive sense, single stranded plant RNA viruses (Tobacco etch virus, Cauliflower mosaic virus, Tobacco mosaic virus etc). In this method, the antigenic determinant is engineered into a plant virus capsid gene. This virus can infect susceptible host plant initiating intracellular production and accumulation of the epitope. The foreign DNA and the viral genome will not become integrated into host plant genome and thus are expressed by infected generation only (Walmsley and Arntzen, 2000; Yusibov et al., 1997). In addition, transient expression of heterologous proteins in intact leaves was demonstrated by infiltration (agroinfiltration technique) of suspensions of A. tumefaciens harbouring a binary vector into leaf interstitial spaces (Kapila et al., 1997). This study put forward a rapid recombinant protein expression technique that is inherently flexible and scalable.
PROTECTIVE MECHANISM OF EDIBLE VACCINES
Mucosal surface is the largest immunologically active tissue in body that lines digestive, respiratory and reproductive tracts (Tacket and Mason, 1999). At the same time, gut is one of the most important locations for development, residence and portal of entry of pathogenic microorganisms into body systems. Gut associated lymphoid tissues (GALT) are important structures evoking immune response in avian species. GALT resides in the intestinal epithelium, lamina propria and specialized lymphoid structures in intestine. Though birds lack highly structured lymphnodes, lymphoid aggregations carry specialized epithelium with microfold cells (M cells), which can take up gut lumen contents and present them to macrophages and dendritic cells on intestinal wall. B and T lymphocytes are also located in intestinal wall (Davison et al., 2008). Upon oral vaccination with transgenic plants, the candidate antigen protected by bio–encapsulation is released into intestine. The cell wall which protects the antigen from enzymes and secretions of foregut slowly breaks and release cell contents to intestinal lumen. The released antigens are then taken up by M cells that are present on intestinal epithelium. These identified antigens are subsequently passed onto other immune cells. This activates production of specific serum IgG (Ig Y), IgE and local IgA antibodies and memory cells. Ig A is responsible for transient immune response and Ig Y for secondary immune response in birds. These antibodies can act against the pathogen specific antigen on succeeding exposures.
PLANT BASED EDIBLE VACCINES AGAINST POULTRY DISEASES INFECTIOUS BURSAL DISEASE (IBD)
IBD is an economically important viral disease of poultry caused by virus coming under family Birnaviridae which is characterized by a bisegmented double–stranded RNA genome. The most immunogenic protein of IBD virus has been confirmed as VP2. Extensive characterization of this protein has been done and is widely used for the production of subunit vaccines against IBD. Using A. tumefaciens mediated transformation technique, VP2 protein was successfully introduced into Arabidopsis thaliana (Wu et al., 20041). Immunization of chicken with plant origin edible vaccines has shown that IBD oral vaccine can induce a variety of Ig G response and the immune protection was comparable to commercial vaccines (Wu et al., 20042). When birds were orally immunized using VP2 antigen at 1st and 3rd week of age followed by challenge at 4th week, 80% protection level was observed. At the same time, following immunization with a commercial IBD vaccine at 1st week of age and transgenic plant vaccine at 3 weeks of age evoked 90% protection. This was the first report showing protection in chicken with an antigen expressed in plants. Wu et al., (2007) produced host protective antigen VP2 on rice seeds (Oryza sativa). SPF chickens after oral immunization with antigen expressed on rice seeds resulted in production of neutralizing antibodies to counteract pathogen and were protected from highly virulent strains when challenged. In 2013, Taghavian reported that tobacco plant produced via transgenesis mediated by Cauliflower mosaic virus for production of VP2 antigen was as effective as commercial vaccines, in producing immunity in mice. Chen et al., 2012 first reported the process describing the production of an IBDV VP2 epitope vaccine in Chenopodium quinoa using plant virus epitope (Bamboo mosaic virus) presentation system. In 2013, Gomez et al., through agroinfiltration of Nicotiana benthamiana for expression of VP2 protein could obtain 1% of total soluble protein as expressed protein. Oral vaccination with a two–fold concentrate of the extract (12 μg of protein) in three doses was clearly enough to induce neutralizing antibody response in chickens.
AVIAN REO VIRUS INFECTIONS (ARV)
Avian reo virus is a double stranded RNA virus which causes a number of avian diseases like malabsorption syndrome, viral arthritis, chronic respiratory disease etc. Young birds are more susceptible to reo viral infections. The principal approach to control of ARV infections is by vaccination in young birds. The most immunogenic capsid protein of ARV is reported to be σC (Wickramasinghe et al., 1993) and has been used for developing subunit vaccines against ARV. Huang et al., (2006) successfully expressed σC antigen in alfalfa plant through Agrobacterium mediated transgenesis and they found that antigenic protein is present as a monomer in alfalfa plant. Wu et al., (2009) reported that the recombinant σC protein expressed in Arabidopsis thaliana has potential use for extensive vaccination against ARV in large flocks. Transgenic tobacco plants were reported to produce σC protein and the yield was ranging from 0.01– 0.02% of the total soluble proteins (Lu et al., 2011).
NEWCASTLE DISEASE (ND)
Newcastle disease is caused by a single stranded RNA virus belonging to family Paramyxoviridae. Infection of host cells by NDV is by the interaction of surface glycoproteins haemagglutinin–neuraminidase (HN) and fusion protein (F) (Stone–Hulslander and Morrison, 1997). Hahn et al., in 2007 attempted HN antigen production in tobacco plants transformed with A. tumefaciens. Oral vaccination of 6 week old chicken with tobacco expressing HN provided immune protection from NDV infection. In a study conducted by Berinstein et al., (2005), both F and HN antigen were expressed in transgenic potato plant and oral vaccination with expressed proteins resulted in inducing mucosal and systemic immune responses. Dow AgroSciences in 2006 produced the first commercial plant–made vaccine for Newcastle disease in chicken (Internet). Using agroinfiltation techniques, Gomez et al., (2009) increased the yield of expressed HN glycoprotein in transformed (A.tumifaciens mediated) Nicotiana benthamiana plants.
INFECTIOUS BRONCHITIS
Infectious bronchitis is an acute, highly contagious respiratory, renal, and urogenital disease caused by a virus coming under the family Coronaviridae. The disease is characterized by heavy mortality in affected flock. Among the 3 major structural proteins of IBV, cleaved spike S1 glycoprotein is identified to be capable of inducing virus neutralizing and haemagglutination inhibiting antibodies (Moore et al., 1997). The first report of expression of IBV S1 glycoprotein was given by Zhou et al., in 2003. In their study, oral immunization with transgenic potato expressing IBV S1 glycoprotein produced immunity and protection in mice and chicken when challenged with virulent strains. Spike protein expressed in transgenic potato on oral administration produced detectable levels of serum neutralizing antibodies (Zhou et al., 2004).
AVIAN INFLUENZA (AI)
Avian influenza viruses are coming under family Orthomyxoviridae, which have raised global concern due to their effect on poultry populations, their ability to cause serious conditions in human beings and their pandemic potential (WHO, 2014). Kalthoff et al., (2010) analyzed the immunogenic potential of plant expressed full length hemagglutinin (rHA0) of HPAIV (H5N1) in several vaccine formulations within chicken. Transgenic tobacco plant (N. benthamiana) with rHA0 protein evoked marked immune responses with production of neutralizing antibodies in chickens and it produced protective immunity against virus challenge. A transgenic plant (Arabidopsis thaliana) containing HPAIV H5N1 antigen in its endoplasmic reticulum, which can be used as edible vaccine or as diagnostic reagents for avian influenza virus infection, was developed by Hwang et al., (2012). Shoji et al., 2012 reported that HA proteins were expressed on tobacco plant and oral immunization of mice with these HA antigens induced serum antihemagglutinin IgG. Hemagglutination inhibiting antibody confers protection against subsequent infections. Kanakarajan et al., (2012) developed a plant based vaccine against low pathogenic avian influenza in transgenic tobacco plant.
COCCIDIOSIS (Eimeria tenella)
Coccidiosis is caused by intracellular protozoan organisms belonging to the genus Eimeria. Sathish et al., in 2012 expressed the microneme proteins (EtMIC2) of E. tenella in tobacco leaves using Agrobacterium. Oral administration of this plant based vaccine produced protective antibody against Eimeria tenella infections and reduced the oocyst output. Body weight gain of orally immunized chickens was considerably high compared to control.
CHICKEN INFECTIOUS ANAEMIA
Chicken anaemia virus (CAV), a member of Circoviridae family is a single stranded virus with a circular genome. The only structural protein found in CAV is VP1. Hence, it is used as antigen for recombinant vaccine production (Cunningham et al., 2001). Lacorte et al., (2007) expressed 3 proteins including the structural protein VP1 in transgenic tobacco plant and suggested that there is need for optimization of VP1 expression level in transgenic plant before releasing it as commercial edible vaccine.
SHORTCOMINGS AND FUTURE PROSPECTS
Production and marketing of plant based edible vaccines against poultry diseases are still in its infancy. But experimental results are suggesting that commercial preparations of plant based edible vaccines may become an alternative to conventional vaccines in near future. Rigorous research work going on in this area may help to overcome the difficulties like standardization of expressed antigen concentration, vaccine formulation, safety, efficacy and stability under field conditions etc. Despite the fact that plant based vaccines are free of pathogens of human or animal origin, presence of pesticide residues and secondary metabolites or toxins may pose a major problem. Crops that are commonly used in poultry feed may help to overcome these issues to an extent. Repeated administration of mucosal antigens can result in suppression of humoral immune response (Jacob et al., 2013). Standardization of expressed antigen levels is difficult in biological systems like plants. Hence, additional research works are to be conducted to identify the exact mechanism of mucosal and systemic immune response produced by edible vaccines.
CONCLUSION
During the last decade, several research works have been conducted to express candidate antigen in edible part of plants against various diseases including that of poultry. Results obtained from oral immunization trials are quite promising. Commercial preparations of edible vaccines may serve as a better alternative to conventional vaccines in near future. To make it a reality, detailed studies in direction to overcome the technical difficulties are needed.
REFERENCES
Aboul–Ata AE, Vitti A, Nuzzaci N, El–Attar AK, Piazzolla G, Tortorella C, Harandi AM, Olson O, Wright SA, Piazzolla P (2014). Plant–Based Vaccines: Novel and Low–Cost Possible Route for Mediterranean Innovative Vaccination Strategies. Adv. Virus Res. 89: 1 – 37.
PMid:24751193
Alvarez ML, Pinyerd HL, Crisantes JD, Rigano MM, Pinkhasov J, Walmsley AM, Mason HS, Cardineau GA (2006). Plant–made subunit vaccine against pneumonic and bubonic plague is orally immunogenic in mice. Vaccine. 24:2477 – 2490.
http://dx.doi.org/10.1016/j.vaccine.2005.12.057
PMid:16442673
Arakawa T, Chong DKX, Langridge WHR (1998). Efficacy of a food plant–based oral cholera toxin B subunit vaccine. Nature Biotechnol. 16:292 – 297.
http://dx.doi.org/10.1038/nbt0398-292
PMid:9528012
Berinstein A, Vazquez–Rovere C, Asurmendi S, Gomez E, Zanetti F, Zabal O, Tozzini A, Conte GD, Taboga O, Calamante G, Barrios H, Hopp E, Carrillo E (2005). Mucosal and systemic immunization elicited by Newcastle disease virus (NDV) transgenic plants as antigens. Vaccine. 23: 5583 – 5589.
http://dx.doi.org/10.1016/j.vaccine.2005.06.033
PMid:16099555
Bock R (2014). Genetic engineering of the chloroplast: novel tools and new applications. Curr. Opin. Biotechnol. 26:7 – 13.
http://dx.doi.org/10.1016/j.copbio.2013.06.004
PMid:24679252
Chen Q, Lai H (2013). Plant–derived virus–like particles as vaccines. Hum. Vaccin. Immunother. 9(1):26 – 49.
http://dx.doi.org/10.4161/hv.22218
PMid:22995837 PMCid:PMC3667944
Chen TH, Chen TH, Hu CC, Liao JT, Lee CW, Liao JW, Lin MY, Liu HJ, Wang MY, Lin NS, Hsu YH (2012). Induction of protective immunity in chickens immunized with plant–made chimeric Bamboo mosaic virus particles expressing very virulent Infectious bursal disease virus antigen. Virus Res. 166: 109 – 115.
http://dx.doi.org/10.1016/j.virusres.2012.02.021
PMid:22406128
Cummings JF, Guerrero LM, Moon JE, Waterman P, Nielsen RK, Jefferson S, Gross FL, Hancock K, Katz JM, Yusibov V (2014). Safety and immunogenicity of a plant–produced recombinant monomer hemagglutinin–based influenza vaccine derived from influenza A (H1N1) pdm09 virus: A Phase 1 dose–escalation study in healthy adults. Vaccine. 32: 2251 – 2259.
http://dx.doi.org/10.1016/j.vaccine.2013.10.017
PMid:24126211
Cunningham SC, Lew AM, Tannock GA (2001). The antigenicity of the chicken anemia virus protein VP3 (Apoptin). Avian Pathol. 30: 613–619.
http://dx.doi.org/10.1080/03079450120092107
PMid:19184955
Curtiss RI, Cardineau CA (1990). Oral immunisation by transgenic plants, World Patent Application. WO 90/02484.
Dalsgaard K, Uttenthal A, Jones TD, Xu F, Merryweather A, Hamilton WDO, Langeveld JPM, Boshuizen RS, Kamstrup S, Lomonossoff GP (1997) Plant–derived vaccine protects target animals against a viral disease. Nat Biotechnol. 15:248 – 252.
http://dx.doi.org/10.1038/nbt0397-248
PMid:9062924
Daniell H, Khan MS, Allison L (2002). Milestones in chloroplast genetic engineering: an environmental friendly era in biotechnology. Trends Plant Sci. 7:84–91.
http://dx.doi.org/10.1016/S1360-1385(01)02193-8
Daniell H, Muthukumar B, Lee SB (2001). Marker free transgenic plants: engineering the chloroplast genome without the use of antibiotic resistance genes. Curr Genet. 39:109 – 16.
http://dx.doi.org/10.1007/s002940100185
PMid:11405095
Dhama K, Wani MY, Deb R, Karthik K, Tiwari R, Barathidasan R, Kumar A, Mahima, Verma AM, Singh SD (2013). Plant based oral vaccines for human and animal pathogens– a new era of prophylaxis: current and future perspectives. Journal of Experimental Biology and Agricultural Sciences. 1 (1).
Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB (2011). Clinical applications of DNA vaccines: current progress. Vaccines. DOI: 10.1093/cid/cir334.
http://dx.doi.org/10.1093/cid/cir334
Gelvin SB (2003). Agrobacterium–mediated plant transformation: the biology behind the gene–jockeying tool. Microbiol. Mol. Biol. Rev. 67:16–37.
http://dx.doi.org/10.1128/MMBR.67.1.16-37.2003
PMid:12626681 PMCid:PMC150518
Gomez E, Lucero MS, Zoth SC, Carballeda JM, Gravisaco MJ, Berinstein A (2013). Transient expression of VP2 in Nicotiana benthamiana and its use as a plant–based vaccine against Infectious Bursal Disease Virus. Vaccine. 31: 2623 – 2627.
http://dx.doi.org/10.1016/j.vaccine.2013.03.064
PMid:23583894
Gomez E, Zoth SC, Asurmendi S, Rovere CV, Berinstein A (2009). Expression of Hemagglutinin–Neuraminidase glycoprotein of Newcastle Disease Virus in agroinfiltrated Nicotiana benthamiana plants. J. Biotechnol. 144(4): 337 – 340.
http://dx.doi.org/10.1016/j.jbiotec.2009.09.015
PMid:19799942
Gorantala J, Grover G, Rahi A, Chaudhary P, Rajwanshi R, Sarin LB, Bhatnagar R (2014). Generation of protective immune response against anthrax by oralimmunization with protective antigen plant–based vaccine. J. Biotechnol. 176: 1 – 10.
http://dx.doi.org/10.1016/j.jbiotec.2014.01.033
PMid:24548460
Hahn BS, Jeon IS, Jung YJ, Kim JB, Park JS, Ha SH, Kim KH, Kim HM, Yang JS, Kim YH (2007). Expression of hemagglutinin–neuraminidase protein of Newcastle disease virus in transgenic tobacco. Plant. Biotechnol. Rep. 1:85 – 92.
http://dx.doi.org/10.1007/s11816-007-0012-9
Haq TA, Mason HS, Clements JD, Arntzen CJ (1995). Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science. 268:714 – 716.
http://dx.doi.org/10.1126/science.7732379
PMid:7732379
Hernandez M, Cabrera–Ponce JL, Fragoso G, Lopez–Casillas F, Guevara–Garcia A, Rosas G (2007). A new highly effective anticysticercosis vaccine expressed in transgenic papaya. Vaccine. 25:4252 – 60.
http://dx.doi.org/10.1016/j.vaccine.2007.02.080
PMid:17399859
Hernandez M, Cabrera–Ponce JL, Fragoso G, López–Casillas F, Guevara–Garcia A, Rosas G (2008). A new highly effective anticysticercosis vaccine expressed in transgenic papaya. Vaccine. 25:4252 – 60.
http://dx.doi.org/10.1016/j.vaccine.2007.02.080
PMid:17399859
Huang LK, Liao SC, Chang CC, Liu HJ (2006). Expression of avian reovirus σC protein in transgenic plants. J. Virol. Methods. 134: 217 – 222.
http://dx.doi.org/10.1016/j.jviromet.2006.01.013
PMid:16488486
Hwang HI, Sohn EJ, Kwon H, Na J, Jeon EH, Im SJ, Park KS, Sung YC (2012). United States Patent Application. Pub. No.: US 2012/0219580 A1.
Jacob SS, Cherian S, Sumithra TG, Raina OK, Sankar M (2013). Edible vaccines against veterinary parasitic diseases–Current status and future prospects. Vaccine. 31: 1879 – 1885.
http://dx.doi.org/10.1016/j.vaccine.2013.02.022
PMid:23485715
Kalthoff D, Giritch A, Geisler K, Bettmann U, Klimyuk V, Hehnen HR, Gleba Y, Beer M (2010). Immunization with plant–expressed hemagglutinin protects chickens from lethal highly pathogenic avian influenza virus H5N1 challenge infection. J. Virol. 84(22):12002 – 12010.
http://dx.doi.org/10.1128/JVI.00940-10
PMid:20810729 PMCid:PMC2977904
Kanakarajan S, Tolf C, Lundgren A, Waldenstrom J, Brodelius PE (2012). Transient Expression of Hemagglutinin Antigen from Low Pathogenic Avian Influenza A (H7N7) in Nicotiana benthamiana. Plos one. DOI: 10.1371/journal.pone.0033010.
http://dx.doi.org/10.1371/journal.pone.0033010
Kapila J, DeRycke R, Montagu VM, Angenon G (1997). An Agrobacterium–mediated transient gene expression system for intact leaves. Plant Sci. 122:101 – 108.
http://dx.doi.org/10.1016/S0168-9452(96)04541-4
Klein NP, Bartlett J, Fireman B, Rowhani–Rahbar A, Baxter R (2013). Comparative effectiveness of acellular versus whole–cell pertussis vaccines in Teenagers. Pediatrics. doi:10.1542/peds.2012 – 3836.
Kohli A, Leech M, Vain P, Laurie DA, Christou P (1998). Transgene organization in rice engineered through direct DNA transfer supports a two–phase integration mechanism mediated by the establishment of integration hot spots. Proc. Natl. Acad. Sci. 95:7203 – 7208.
http://dx.doi.org/10.1073/pnas.95.12.7203
PMid:9618563 PMCid:PMC22782
Kumar CS, Deepesh G, Mahavir Y, Archana T (2012). Edible vaccine: a new platform for the development of malaria vaccine. Crit. Rev. Eukaryot. Gene Expr. 22(3):243 – 248.
http://dx.doi.org/10.1016/j.vaccine.2012.07.072
http://dx.doi.org/10.4161/hv.18862
Lacorte C, Lohuis H, Goldbach R, Prins M (2007). Assessing the expression of chicken anemia virus proteins in plants. Virus Res. 129(2): 80 – 86.
http://dx.doi.org/10.1016/j.virusres.2007.06.020
PMid:17698236
Legocki AB, Miedzinska K, Czaplin ska M, Płucieniczak A, Wedrychowicz H (2005). Immunoprotective properties of transgenic plants expressing E2 glycoprotein from CSFV and cysteine protease from Fasciola hepatica. Vaccine. 23:1844 – 1846.
http://dx.doi.org/10.1016/j.vaccine.2004.11.015
PMid:15734053
Lindh I, Brave A, Hallengard D, Hadad R, Kalbina I, Strid A, Andersson S (2014). Oral delivery of plant–derived HIV–1 p24 antigen in low doses shows a superior priming effect in mice compared to high doses Ingrid. Vaccine. 32: 2288 – 2293.
http://dx.doi.org/10.1016/j.vaccine.2014.02.073
PMid:24631072
Lossl AG, Waheed MT (2011). Chloroplast–derived vaccines against human diseases: achievements, challenges and scopes. Plant Biotechnol. J. 9: 527 – 539.
http://dx.doi.org/10.1111/j.1467-7652.2011.00615.x
PMid:21447052
Lou XM, Yao QH, Zhang Z, Peng RH, Xiong AS, Wang HK (2007). Expression of the human hepatitis B virus large surface antigen gene in transgenic tomato plants. Clin. Vaccine Immunol. 14: 464 – 469.
http://dx.doi.org/10.1128/CVI.00321-06
PMid:17314228 PMCid:PMC1865599
Lu SW, Wang KC, Liu HJ, Chang CD, Huang HJ, Chang CC (2011). Expression of avian reovirus minor capsid protein in plants. J. Virol. Methods. 173:287 – 93.
http://dx.doi.org/10.1016/j.jviromet.2011.02.021
PMid:21354211
Maliga P, Bock R (2011). Plastid biotechnology: food, fuel, and medicine for the 21st century. Plant Physiol. 155:1501 – 1510.
http://dx.doi.org/10.1104/pp.110.170969
PMid:21239622 PMCid:PMC3091108
Martinz RDC, Irache JM, Gamazo C (2012). Acellular vaccines for ovine brucellosis – a safer alternative against a worldwide disease. Expert Rev. Vaccines. 11(1):87 – 95.
http://dx.doi.org/10.1586/erv.11.172
PMid:22149711
Mason HS, Haq TA, Clements JD, Arntzen CJ (1998). Edible vaccine protects mice against Escherichia coli heat–labile enterotoxin (LT): potatoes expressing a synthetic LT–B gene. Vaccine. 16(13):1336 – 1343.
http://dx.doi.org/10.1016/S0264-410X(98)80020-0
Mason HS, Warzecha H, Mor T, Arntzen CJ (2002). Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 8:324 – 329.
http://dx.doi.org/10.1016/S1471-4914(02)02360-2
Moffat AS (1995). Exploring transgenic plants as a new vaccine source. Science. 268:658 – 660.
http://dx.doi.org/10.1126/science.7732373
PMid:7732373
Moore KM, Jackwood MW, Hilt DA (1997). Identification of amino acids involved in a serotype and neutralization specific epitope within the S1subunit of avian infectious bronchitis virus. Arch. Virol. 142:2249 – 2256.
http://dx.doi.org/10.1007/s007050050239
PMid:9672590
Muller CP, Marquet–Blouin E, Fack F, Damien B, Steinmetz A, Bouche FB (2003). Immunogenic measles antigens expressed in plants: role as an edible vaccine for adults. Vaccine. 21(7–8):816 – 819.
http://dx.doi.org/10.1016/S0264-410X(02)00606-0
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T (2007). Rice–based mucosal vaccine as a global strategy for cold–chain and needle–free vaccination. Proc. Natl. Acad. Sci. 104: 10986 – 10991.
http://dx.doi.org/10.1073/pnas.0703766104
PMid:17573530 PMCid:PMC1904174
Pniewski T (2013). The twenty–year story of a plant–based vaccine against hepatitis B: stagnation or promising prospects. Int. J. Mol. Sci. 14: 1978 – 1998.
http://dx.doi.org/10.3390/ijms14011978
PMid:23337199 PMCid:PMC3565360
Roy S, Tyagi A, Tiwari S, Singh A, Sawant SV, Singh PK, Tuli R (2010). Rabies glycoprotein fused with B subunit of cholera toxin expressed in tobacco plants folds into biologically active pentameric protein. Protein Expres. Purif. 70: 184 – 190.
http://dx.doi.org/10.1016/j.pep.2009.10.002
PMid:19818857
Rubio EL, Anaya ER, Lopez J, Florez MTO, Lim MG (2012). Induction of a protective immune response to rabies virus in sheep after oral immunization with transgenic maize, expressing the rabies virus glycoprotein. Vaccine. 30: 5551 – 5556.
http://dx.doi.org/10.1016/j.vaccine.2012.06.039
PMid:22749836
Sala F, Rigano MM, Barbante A, Basso B, Walmsley AM, Castiglione S (2003). Vaccine antigen production in transgenic plants: strategies, gene constructs and perspectives. Vaccine. 21: 803 – 808.
http://dx.doi.org/10.1016/S0264-410X(02)00603-5
Sathish K, Sriraman R, Subramanian BM, Rao NH, Kasa B, Donikeni J (2012). Plant expressed coccidial antigens as potential vaccine candidates in protecting chicken against coccidiosis. Vaccine. 30:4460 – 4464.
http://dx.doi.org/10.1016/j.vaccine.2012.04.076
PMid:22554463
Shoji Y, Farrance CE, Bautista J, Bi H, Musiychuk K, Horsey A, Park H, Jaje J, Green BJ, Shamloul M, Sharma S, Chichester JA, Mett V, Yusibov V (2012). A plant–based system for rapid production of influenza vaccine antigens. Influenza Other Respir. Viruses. 6: 204 – 210.
http://dx.doi.org/10.1111/j.1750-2659.2011.00295.x
PMid:21974811
Stone–HulslanderJ, Morrison TG (1997). Detection of an interaction between the HN and F proteins in Newcastle disease virus infected cells. J. Virol. 71:6287 – 6295.
PMid:9261345 PMCid:PMC191901
Streatfield SJ (2005). Delivery of plant–derived vaccines. Expert Opin. Drug Deliv. 2:719 – 728.
http://dx.doi.org/10.1517/17425247.2.4.719
PMid:16296796
Tacket CO, Mason HS (1999). A review of oral vaccination with transgenic vegetables. Microb. Infect.1:777–783.
http://dx.doi.org/10.1016/S1286-4579(99)80080-X
Taghavian O (2013). Expression and characterization of infectious bursal disease virus protein for poultry vaccine development and application in nanotechnology. PhD Dissertation submitted to Aachen University, Iran.
Taylor NJ, Fauquet CM (2002). Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 21: 963–977.
http://dx.doi.org/10.1089/104454902762053891
PMid:12573053
Tregoning J, Maliga P, Dougan G, Nixon PJ (2004). New advances in the production of edible plant vaccines: chloroplast expression of a tetanus vaccine antigen, TetC. Phytochem. 65(8):989 – 994.
http://dx.doi.org/10.1016/j.phytochem.2004.03.004
PMid:15110679
Walmsley AM, Arntzen CJ (2000). Plants for delivery of edible vaccines. Curr. Opin. Biotechnol.11:126 – 129.
http://dx.doi.org/10.1016/S0958-1669(00)00070-7
Walmsley AM, Arntzen CJ (2003). Plant cell factories and mucosal vaccines. Curr. Opin. Biotechnol. 14:145 – 50.
http://dx.doi.org/10.1016/S0958-1669(03)00026-0
Wang J, Yu C, Yin X, Zhang W, Qian C, Song L (2011). Monitoring specific antibody responses against the hydrophilic domain of the 23 kDa membrane protein of Schistosoma japonicum for early detection of infection in sentinel mice. Parasit. Vectors. 4:1 – 9.
http://dx.doi.org/10.1186/1756-3305-4-172
PMid:21906319 PMCid:PMC3180346
Wang Y, Deng H, Zhang X, Xiao H, Jiang Y, Song Y, Fang L, Xiao S, Zhen Y, Chen H (2009). Generation and immunogenicity of Japanese encephalitis virus envelope protein expressed in transgenic rice. Biochem. Biophys. Res. Commun. 380(2):292 – 297.
http://dx.doi.org/10.1016/j.bbrc.2009.01.061
PMid:19166811
Wickramasinghe R, Meanger J, Enriquez CE, Wilcox GE (1993). Avian reovirus proteins associated with neutralization of virus infectivity. Virology. 194: 688 – 696.
http://dx.doi.org/10.1006/viro.1993.1309
PMid:8503182
Widorovitz A, Carrillo C, Dus Santos MJ, Trono K, Peralta A, Gomez MC, Rios RD, Franzone PM, Sadir AM, Escribano JM. Borca MV (1999). Induction of a protective antibodyresponse to foot and mouth disease virus in mice followingoral or parenteral immunization with alfalfa transgenic plantsexpressing the viral structural protein VP1. Virology. 255: 347 – 353.
http://dx.doi.org/10.1006/viro.1998.9590
Widorovitz A, Mozovoj M, Santos M, Parreno V, Gomez C, Perez–Filgueira D, Trono K, Rios R, Franzone P, Fernandez F, Carrillo C, Babiuk L, Escribano J, Borca M (2004). Protectivelactogenic immunity conferred by an edible peptide vaccine to bovine rotavirus produced in transgenic plants. J. Gen. Virol. 85: 1825 – 1832.
http://dx.doi.org/10.1099/vir.0.19659-0
PMid:15218166
Wu H, Singh NK, Gunn KS, Giambrone JJ (2009). Research towards development of an edible vaccine for avian reovirus. Avian Dis. 53:376 – 381.
http://dx.doi.org/10.1637/8589-011309-Reg.1
PMid:19848075
Wu H, Singh NK, Locy RD, Gunn KS, Giambrone JJ (20042). Expression of immunogenic VP2 protein of Infectious Bursal Disease Virus Expressed in Arabidopsis thaliana. Biotechnol. lett. 26: 787 – 792.
http://dx.doi.org/10.1023/B:BILE.0000025878.30350.d5
PMid:15269548
Wu H, Singh NK, Locy RD, Gunn KS, Giambrone JJ. 20041. Immunization of Chickens with VP2 Protein of Infectious Bursal Disease Virus Expressed in Arabidopsis thaliana. Avian Dis. 48(3):663 – 668.
http://dx.doi.org/10.1637/7074
PMid:15529992
Wu J, Yu L, Li L, Hu J, Zhou J, Zhou X (2007). Oralimmunization with transgenic rice seeds expressing VP2 protein of infectious bursal disease virus induces protective immune responses in chickens. Plant Biotechnol. J. 5:570 – 578.
http://dx.doi.org/10.1111/j.1467-7652.2007.00270.x
PMid:17561926
Yan–ju YE, Wen–gui LI (2010) Immunoprotection of transgenic alfalfa (Medicago sativa) containing Eg95–EgA31 fusion gene of Echinococcus granulosus against Eg protoscoleces. J. Trop. Med. 3:10 – 13.
Youm JW, Jeon JH, Kim H, Kim YH, Ko K, Joung H, Kim HS (2008). Transgenic tomatos expressinghuman beta–amyloid for use as a vaccine against Alzheimer's disease. Biotechnol. lett. 30: 1839 – 1845.
http://dx.doi.org/10.1007/s10529-008-9759-5
PMid:18604480 PMCid:PMC2522325
Yusibov V, Modelska A, Steplewski K (1997). Antigens produced in plants by infection with chimeric plant viruses immunize against rabies and HIV. Proc. Natl. Acad. Sci. 94: 5784 – 5788.
http://dx.doi.org/10.1073/pnas.94.11.5784
PMid:9159151 PMCid:PMC20857
Yusibov V, Streatfield S J, Kushnir N (2011). Clinical development of plant–produced recombinant pharmaceuticals: Vaccines, antibodies and beyond. Hum. Vaccin. 7(3):313 – 321.
http://dx.doi.org/10.4161/hv.7.3.14207
PMid:21346417
Zhang H, Liu M, Li Y, Zhao Y, He H, Yang G, Zheng C (2010). Oral immunogenicity and protective efficacy in mice of a carrot–derived vaccine candidate expressing UreB subunit against Helicobacter pylori. Protein Expr. Purif. 69(2):127 – 131.
http://dx.doi.org/10.1016/j.pep.2009.07.016
PMid:19651219
Zhang X, Buehner NA, Hutson AM, Estes MK, Mason HS (2006). Tomato is a highly effective vehicle for expression andoral immunization with Norwalk virus capsid protein. Plant Biotechnol. J. 4: 419 – 432.
http://dx.doi.org/10.1111/j.1467-7652.2006.00191.x
PMid:17177807
Zhou JY, Cheng LQ, Zheng XJ, Wu JX, Shang SB, Wang JY, Chen JG (2004). Generation of the transgenic potato expressing full length spike protein of Infectious Bronchitis virus. J. biotechnol. 111: 121–130.
http://dx.doi.org/10.1016/j.jbiotec.2004.03.012
PMid:15219399
Zhou JY, Wu JX, Cheng LQ, Zheng XJ, Gong H, Shang SB, Zhou EM (2003). Expression of Immunogenic S1 Glycoprotein of Infectious Bronchitis Virus in Transgenic Potatoes. J. Virol. 9090 – 9093.
http://dx.doi.org/10.1128/JVI.77.16.9090-9093.2003
PMid:12885926 PMCid:PMC167223