Advances in Animal and Veterinary Sciences
Research Article
Influence of Ginger and Garlic Supplementation on Growth Performance, Whole Body Composition and Oxidative Stress in the Muscles of Nile Tilapia (O. Niloticus)
Rania Mahmoud1, Abeer Aziza1*, Basma Marghani2, Rasha Eltaysh3
*1Department of Nutrition and Nutritional Deficiency Diseases, Faculty of Veterinary Medicine, Mansoura University, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt;3 Department of Pharmacology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt.
Abstract | An experiment was conducted to determine the impact of using garlic and ginger powder on growth performance, body composition, lipid peroxidation and antioxidant enzymes activities in muscle tissues of Nile tilapia fingerlings. Three isonitrogenous (32%) and isocaloric (3000 kcal DE) diets were formulated , control basal diet, diet supplemented with 1.5% ginger powder, other diet supplemented with 1.5% garlic powder and fed to the fish for sixty days at 3% body weight. No significant effects were found in final body weight (FBW) between experimental groups of fish. Body weight gain (BWG) and specific growth rate (SGR) were significantly (p≤ 0.05) decreased in Nile tilapia fish fed diets supplemented with garlic and ginger powder compared to the control group. Also, there was improvement of feed conversion ratio (FCR) of Nile tilapia fish fed control basal diet compared with other experimental groups. No significant differences in proximate chemical composition of whole body of fish between experimental groups. Lipid peroxidation (malondialdehyde, MDA) in muscle tissues of fish groups fed diets supplemented with ginger and garlic (1.5%), respectively, showed a significant (p≤ 0.05) decrease in MDA levels. Also, superoxide dismutase (SOD) was significantly (p≤ 0.05) increase in fish group fed diet supplemented with garlic compared with other experimental groups. No significant differences of Catalase (CAT) and reduced glutathione (GSH) of fish muscle of experimental groups. To sum up, adding garlic and ginger at 1.5% had no significant effect on Nile tilapia growth performance, body composition, while using of garlic as a feed additive significantly reduce lipid peroxidation and had antioxidant effect.
Keywords | Ginger, Garlic, Nile tilapia, Growth performance, Oxidative stress
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 21, 2018; Accepted | December 18, 2018; Published | March 25, 2019
*Correspondence | Abeer Aziza, Department of Nutrition and Nutritional Deficiency Diseases, Faculty of Veterinary Medicine, Mansoura University, Egypt; Email: [email protected]
Citation | Mahmoud R, Aziza A, Marghani B, Eltaysh R (2019).Influence of ginger and garlic supplementation on growth performance, whole body composition and oxidative stress in the muscles of nile tilapia (o. niloticus) Adv. Anim. Vet. Sci. 7(5): 397-404.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.5.397.404
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Mahmoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In the recent decades, aquaculture production has greatly increased (FAO, 2014). Among the Tilapias, Nile tilapia, Oreochromis niloticus, is one of the most prominent and commonly cultured species. It has some advantageous features for culture such as rapid growth, good survival in high-density culture, disease resistance and ability to reproduce in captivity and high adaptation to a wide extent of supplemented nutrients (Gao and Lee, 2012).
There is large number of feed additives available to enchance fish growth performance. Some of antibiotics and hormones are used as feed additives for fish therefore, they may induce harmful side effects. The use of antibiotic growth promoters (AGPs) as feed additives in the aquaculture industry has been criticised by government policies and customers owing to accessible development of microorganism resistance to those products and their potential harmful effects on human health (Baruah et al., 2008). World Health Organization encourages using of medicinal herbs and plants to substitute or minimize the utilization of chemicals through the worldwide trend to go back to the nature. Tries to use the natural materials like medicinal plants may be wide accepted as feed additives to reinforce potency of feed utilization and animal productive performance (Levic et al., 2008).
Ginger and garlic are spices, in addition to contributing taste and aroma to foods, in addition contain a kind of bioactive substances that are of considerable use from the point of view of food science and technology (Yanishlieva et al., 2006). Additionally, use of plant extracts as natural antioxidants has gained increasing interest due to the worldwide trend of restriction in use of artificial substances, also antioxidant rich plant extracts have potential benefits in food preservations (Uhar et al., 2006).
It has been reported that ginger has potential benefits in aquaculture management such as growth enhancement and immunomodulatory agent in fish and control an Aeromonas hydrophila infection especially in rainbow trout (Masoud and Mostafa, 2013; Nya et al., 2009). Moreover, Ginger contains alkaloids, flavonoids, polyphenols, saponin, steroids, tannin, fiber, carbohydrate, vitamins, carotenoids and minerals (Otunola et al., 2010).
It has been reported that dietary garlic as a growth promoter in Nile tilapia (Oreochromis niloticus) improved weight gain, feed intake and feed efficiency (Abdel-Hakim et al., 2010). In aquacultural operations, garlic promotes growth, enhances immunity, stimulates appetency and strengthens the management of bacterial and fungal pathogens additionally hematological and serum biochemical parameters impact (Mehri et al., 2014). Most aquatic garlic researches have involved fresh garlic extracts, with experimental subjects either fed a garlic-added feed or treated with a garlic juice immersion. Allicin is that the most active principle in garlic that directly and actively kills parasites (Adler et al., 1997).
Lipids peroxidation (LPO) is that the process, in which lipids are oxidized through peroxides formation (LPO). Aquatic organisms have a higher quantity of lipids and residues of polyunsaturated fatty acid that is a substrate for oxidation. One of the final products of LPO is malondialdehyde (MDA), therefore it is usually used for observance LPO (Lushchak, 2011). To avoid oxidative damage, organisms have an anti-oxidative system like superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) (Pi et al., 2010). So, changes inside the activities of antioxidant enzymes like catalase, glutathione peroxidase and superoxide dismutase depicts oxidative stress (Ullah, 2015).
Therefore, this experimental work aimed to investigate the effects of using ginger and garlic at level 1.5% as feed additives on some nutritional and physiological parameters of Nile tilapia (O. niloticus) fingerlings for sequential 60 days.
MATERIALS AND METHODS
Preparation of the Food Additives
Fresh ginger (Zingiber officinale, Egyptian yellow ginger) and fresh garlic bulbs (Allium sativum, Egyptian white garlic) were procured from a local market. The dry skin of both fresh garlic and ginger were removed before use; they were peeled and cut into small pieces then dried in oven at 50 Co for 14 hours at the department of pharmacology, Faculty of Veterinary Medicine, Mansoura University, according to (Ajiboye et al., 2016). The dried ginger and garlic were crushed finely by employing a hand held kitchen utensil and kept in dry containers till formulation and preparation of experimental diet.
Formulation of Experimental Diets
Three isonitrogenous (32%) and isocaloric (3000 Kcal DE/kg) diets were formulated at the department of Nutrition and nutritional deficiency diseases, Faculty of Veterinary Medicine, Mansoura University, to give the nutrient requirements of Nile tilapia (O. niloticus) according to (NRC, 2011). Ginger and garlic powder were added to the basal diet at 1.5% to formulate the experimental diets. Composition of the control basal diet & experimental diets are listed in (Table 1).
Experimental Fish and Housing
Nile tilapia, Oreochromis niloticus were obtained from khafr Elsheihk, Egypt. Fish were conveyed by plastic bags (50-liter) filled with water and oxygen to animal nutrition department. Prior to the beginning of the experiment, fish were adapted to the experimental conditions and fed basal diet twice daily for 15 days. After fish adaptation, one hundred and twenty fish with an average body weight of (10g) were randomly stocked in 6 glass aquaria (80 cm length, 35 cm width, 40 cm height) represented to three groups of fish, 20 fish in each. Three experimental diets were used, diet 1 (control), diet 2 (basal diet supplemented with ginger 1.5%) and diet 3 (basal diet supplemented with garlic 1.5%). Each diet was fed to one group of fish in duplicate aquariumat. Fish fed experimental diets at 3% body weight (Elliott, 1975) twice daily (10.00 h-14.00 h) for six days a week and persisted for consecutive 60 days. Daily cleanup for each aquarium was carried out with partial replacement of water by previously stored (for 48 hours) dechlorinated tap water.
Daily feed consumption for each 2 weeks was recorded. Dietary allowances of each experimental group was adjusted bi-weekly, after weighting of fish, according to mean body weight, then placed in small plastic bags for each aquarium for daily feeding of fish.
Table 1: Ingredients and composition of the experimental diets
| Ingredients | Experimental diets | ||
|
|
Control | Ginger 1.5% | Garlic 1.5% |
| Yellow corn (8.5%) | 22.13 | 20.43 | 20.43 |
| Soybean meal (45.5%) | 20.5 | 20.5 | 20.5 |
| Fish meal | 20 | 20 | 20 |
| Corn gluten | 3 | 3 | 3 |
| Gelatin | 2 | 2 | 2 |
| Vegetable oil | 1 | 1.2 | 1.2 |
| Wheat bran | 29 | 29 | 29 |
|
Vitamin/ minerapremix* |
1 | 1 | 1 |
| Salt | 0.3 | 0.5 | 0.5 |
| Vit c | 0.1 | 0.1 | 0.1 |
| Antioxidant | 0.02 | 0.02 | 0.02 |
| Di calcium phosphate | 0.8 | 0.8 | 0.8 |
| Methionine | 0.15 | 0.15 | 0.15 |
| Ginger | 0.00 | 1.50 | 0.00 |
| Garlic | 0.00 | 0.00 | 1.50 |
| Chemical analysis % | |||
|
Ash |
6.34 | 6.28 | 4.12 |
|
EE |
9.25 | 9.14 | 9.89 |
|
Moisture |
4.51±0.12 | 3.81±0.21 | 4.5±0.11 |
| Crude protein | 32 | 31.7 | 31.8 |
*vitamin mixture supplies the following per kilogram of diet:: vit. A -1,200.000 IU; vit. D3 -200,000 IU; vit. E - 12,000 mg; vit. K3 - 2,400 mg;vit. B1 - 4,800 mg; vit. B2 - 4,800 mg; vit. B6 - 4,000 mg; vit. B12 - 4,800 mg; folic acid - 1,200 mg; vit. C - 48,000 mg; biotin - 48 mg; choline- 65;000 mg; niacin - 24,000 mg; Fe - 10,000 mg; Cu - 600 mg; Mg - 4,000 mg; Zn - 6,000 mg; I - 20 mg; Co - 2 mg; Se - 20 mg.
Samples Collection
At the end of the experimental period, the following growth and feed utilization indices were calculated: body weight gain (BWG), feed conversion ratio (FCR) & specific growth rate (SGR). The calculation formula for relative index is listed below:
BWG= Final weight -Initial weight
FCR (%) = (daily feed intake (g) /wet BWG (g)) × 100
SGR (%) = (Ln WF– Ln WI) × 100/days of feeding
WF and WI were Final and Initial fish weights, respectively.
Body Composition Analysis
At end of the feeding trial, fish samples (4 fish/ aquarium) from all experimental groups were weighted and kept in plastic bags kept in deep freeze until preparation (dried, minced in an electric meat grinder to obtain homogeneous samples) for whole body chemical composition. Proximate chemical composition according to AOAC methods (2005): moisture content after drying in an oven at 105°C till constant weight; crude protein (N = 6.25) by Kjeldahl digestion and distillation after acid digestion; crude lipid extraction in a Soxhlet apparatus by petroleum ether; ash by incineration in a muffle furnace at 550°C for eight to twelve hrs (Table 2).
Table 2: Effect of experimental diets on growth performance of Nile tilapia fish.
| Parameter | Experimental diets | ||
| Control | Ginger 1.5% | Garlic 1.5% | |
| IBW | 21.76 ± 1.07 | 21.56 ± 0.85 |
22.05 ± 0.87 |
| FBW | 68.25 ± 2.35 | 64.17± 2.40 | 66.18 ± 2.72 |
| BWG |
47.50 ± 0.87a |
43.78± 0.83b |
44.42 ± 1.8b |
| FCR |
1.8 ± 0.030b |
2.12 ± 0.056 a |
2.13 ± 0.13 a |
| SGR |
1.99 ± 0.037 a |
1.59 ± 0.014 b |
1.60 ± 0.023 b |
Values are Means ± SE. SEM = Standard error of means.
Values with different small superscript letters in the same row are significant at P ≤ 0.05.
Tissue Preparation
At the end of the experiment, the frozen muscles were removed from each fish sample and muscle tissues homogenate were prepared at the department of Physiology, Faculty of Veterinary Medicine, Mansoura University, according to (Chen et al., 2012), where 0.5 g of each muscle homogenization was was carried out by a solution formed from 5 mL of 0.1 M potassium phosphate buffer (pH 6.5), centrifuged at 3000 rpm/5 min; then, the supernatant was aspirated, collected into separate Eppendorf tubes and stored at -20ºC for further biochemical analysis of muscle MDA, SOD, CAT and GSH.
Biochemical Analysis of Mda and Antioxidant Enzymes Activities
The biochemical levels of MDA, SOD, GSH and CAT were determined colorimetrically by using commercial kits provided by Bio-diagnostic Company, Egypt. MDA was determined according to (Satoh 1978). Furthermore, SOD, GSH and CAT enzymatic activity in tissue was detected according to Nishikimi et al. (1972), Beutler et al. (1963) and Cohen et al. (1970), respectively..
Statistical Analysis
Statistical analysis of data obtained from the experiment were carried out by software SPSS program package version 17 (SPSS, 2004), using the one-way analysis of variance ANOVA. All data were expressed as means ± SEM. Differences between means were tested for significance by using Duncan’s Range Test (DMRT) as described by (Duncan, 1955). Results were considered significant only at the level of (p ≤ 0.05).
RESULTS
Performance
Effect of using two different feed additives on growth performance of Nile tilapia fish is present in (Table 2). There were significant (p ≤ 0.05) improvement in BWG, FCR and SGR of Nile tilapia fish fed basal control diet compared with other experimental groups. However, there were no significant differences in FBW between fish fed 1.5% ginger and garlic and basal control groups. FCR of Nile tilapia fed control diet was significantly lower than FCR of Nile tilapia fish fed diet supplemented with garlic and ginger.
Proximate Chemical Composition of Whole Fish
The proximate chemical composition of whole fish carcass of experimental groups was shown in (Table 3). The moisture content of whole body carcass of fish fed basal control diet was significantly higher than other groups fed experimental diets. There were no significant differences in crude protein, ether extract content between control and experimental groups. However, there was significant increase of ash of whole fish fed 1.5% ginger than those fed control or 1.5% garlic.
Table 3: Effects of supplementation of ginger or garlic (1.5%) on whole body composition of fingerling Nile tilapia.
| Parameter | Experimental diets | ||
| Control | Ginger 1.5% | Garlic 1.5% | |
| Crude protein | 58.2 ± 0.1 | 58.5 ± 0.1 | 59.15 ± 0.35 |
| Ash |
19.24 ± 0.33b |
21.23± 0.25 ab |
19.5 ± 0.59 b |
| EE | 16.79 ± 0.62 | 16.13± 0.19 | 17.01 ± 1.6 |
| Moisture |
76.86 ± 0.88 ab |
74.91 ± 0.70b |
73.60 ± 0.60 b |
Values are Means ± SE. SEM = Standard error of means.
Values with different small superscript letters in the same row are significant at P ≤ 0.05.
Lipid Peroxidation and Antioxidant Enzyme Activities
Determination of lipid peroxidation (MDA) concentration and antioxidant enzymes in muscle tissue of Nile tilapia fed three experimental diets for 60 days revealed a significant reduction (p ≤ 0.05; p = 0.000) of MDA in fish feeding diet containing ginger and garlic 1.5% respectively when compared to group of fish feeding control diet. Nile tilapia fish fed diet containing garlic 1.5% induce a significant increase (p ≤ 0.05; p = 0.003) in SOD level when compared to groups of fish feeding control diet and diet containing ginger 1.5%. However, There were no significant differences (p>0.05) in CAT and GSH enzymes between control group and other experimental groups (Table 4 and Figure 1).
Table 4: Lipid peroxidation (MDA) & antioxidant enzymes activities in muscle tissues of Nile tilapia fed experimental diets.
| Parameter | Experimental diets | ||
| Control | Ginger 1.5% | Garlic 1.5% | |
|
MDA (n mol/g) |
36.83 ± 1.41 a |
23.90 ± 1.63 b |
23.19 ± 0.11c |
|
SOD (U/g.tissue) |
3.72 ±1.24b |
3.80± 1.55 b |
5.31 ± 1.68 a |
|
CAT (U/g.tissue) |
9.11 ± 1.15 | 10.20± 1.17 | 9.46 ± 0.45 |
|
GSH (mg/g.tissue) |
2.51 ± 0.24 | 2.87± 0.45 | 3.13 ± 0.07 |
Values are Means ± SE. SEM = Standard error of means.
Values with different small superscript letters in the same row are significant at P ≤ 0.05.
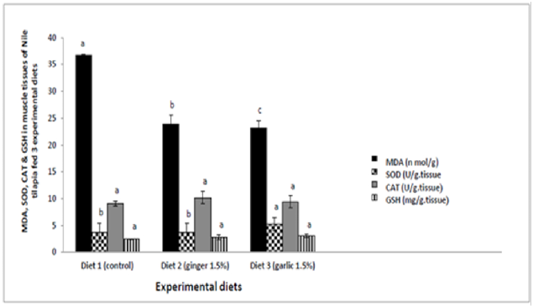
Figure 1: Lipid peroxidation (MDA) and antioxidant enzymes activites in muscle tissues of Nile tilapia fed experimental diets
DISCUSSION
There were no significant difference in FBW between fish fed 1.5% ginger & garlic and basal control group. However, FCR and SGR of Nile tilapia fed 1.5% garlic powder significantly decreased compared with those fed control diet. The present findings were agreement with Labrador et al. (2016) who established that garlic powder supplemented diet (2, 4 or 6%) did not have any significant effect on final weight (g) and weight gain (%), final weight (g) of L. vannamei in comparison to shrimp fed the control diet. In addition, Nwabueze (2012) reported that garlic-supplementation to the diet of Clarias gariepinu did not have any significant effect on BWG. However, Sahu et al. (2007) showed that FCR and SGR were not significantly different in fish (Labeo rohita) fed diet with 0.5% and 1% garlic in comparison to control group. Our results are not in accordance with the results reported by Metwally (2009) who found that the best performance was attained in Nile tilapia fed diet with 3.2% GP. With the same concept, Diab et al. (2002) mentioned feeding diet with 2.5% garlic/kg diet resulted in the highest growth performance in O. niloticus. In addition, Lee et al. (2014) showed that adding of garlic powder by level 3% could positively affect growth performance in fingerling starlet sturgeon.
The conflicting results in FBW of fish supplemented with garlic may be attributed to dose, period of study. This confirmed by the results recorded by Temitope (2012) who concluded that growth rate of T. zillii fed the diets having 20g inclusion level of garlic/kg basal diet had higher growth rate than other group fed (0, 5, 10, 15 g garlic kg-1 basal diet). Furthermore, Aly and Mohamed (2010) examined the growth rates of Nile tilapia when feeding with garlic (10 and 20 g/kg diet fed), and found statistically non-significant increases after one or 2 month, however a significant rise was achieved only after 8 months, indicating that high doses or a long period was needed to reinforce the growth rate. In addition, Salah et al. (2008) reported that feeding of higher doses of garlic 20 g kg-1 diet for prolonged periods gave better results than 10 g kg-1 diet. With the same line, Shalaby et al. (2006) demonstrated that the best performance and high specific growth rate in Nile tilapia fish was obtained by a diet containing 30 g garlic powder per kilogram diet and improvement in FBW was more prominent at higher incorporation levels.
The results concerned with using 1.5% ginger as feed additive are in agreement with those obtained with Şahan et al. (2016) who demonstrated that although ginger have appetizing, growth-stimulating effects on fish species, had no significant effects on the growth parameters once feeding at 2% of the body weight of the fish at level (0.5%, 0.1%, 1.0%). These results clearly showed that the ginger stimulated fish growth in relation to ginger supplementation in a dose dependent manner. Talpur et al. (2013) suggested that the growth was dose-dependent and highest supplementation of ginger at 5 and 10 g/kg feed was most favourable for the growth and survival of Asian sea bass. However, Iheanacho et al. (2017) reported improvement in BWG & SGR in C. gariepinus juvenile when exposed to different concentrations (0.25, 0.50, 0.75 and 1.0 g/35 mgL) of ginger as compared to the control. Also, Venkataramalingam et al. (2007) found administration of ginger can produce significantly higher weight gain and specific growth rates in Penaeusmonod on post larvae.
Vahedi et al. (2017) demonstrated that the results of adding dietary garlic & ginger on growth performance were conflicted and may be attributed or depend on fish species differences, size, age, sex, feeding program, dose of the additive, precursors in the diet, fish nutritional/physiological status and ambient culturing conditions.
Body composition is a good indicator of the physiological condition of a fish but it is relatively time consuming to measure. In the present study, fish fed control diet had significantly the higher moisture content in the body compared to other groups. There were no significant differences in crude protein; ash & ether extract content between control and experimental groups. Nile tilapia fish group fed diet supplemented with 1.5% ginger had higher ash content. The present findings are partially in agreement with, Ebrahim Dorche et al. (2013) who concluded that ether extract and moisture content were not affected by adding 150 mg garlic essential oil/ kg diet. Additionally , effect of adding garlic on whole body composition of fish are varied and may be attributed to fish species , nutrition program, diet composition, level of garlic extract, its precursors in the diet and environmental conditions (Shalaby et al., 2006). On contrary to our results, Shalaby et al. (2006) observed that the lowest lipid and highest protein content of whole body of (A. ruthensis) fish fed with 30 g garlic powder/kg diet. Farahi et al. (2010) reported that using 1, 2and 3% of garlic in diets of the rainbow trout for 60 days led to significant difference in the percentage of crude protein, fat and ash content in the body of fish compared with control group. Higher body ash content in group of fish fed on diet supplemented with 1.5% ginger may be attributed to the constant access to food, absorbing minerals and nutrients by aquatic organisms (Samadi, 2012).
Based on the results of the present study, (MDA) levels have been used as a potent marker for lipid per oxidation’s (LPOs). There was a significant decrease in (MDA) in the muscle tissues of fishes fed diet supplemented with ginger or garlic 1.5% than fish fed control basal diet. Ginger is considered a potent antioxidant substance which prevents free radicals generation (Kim et al., 2007). Phenolic compounds of ginger (gingerols, shogaols, volatile oils, flavonoids, and phenolic ketone derivatives) encourage antioxidant activity against free radicals and prevent lipid peroxidation (Lebda et al., 2012). The most important active ingredients of Ginger such as gingerdiol, zingibrene, zingerone, gingerols and shogaols are known to possess anti-oxidant activities (Caputi Jambrenghi et al., 2005). It has been also reported that dietary garlic can improve the antioxidant status of rainbow trout (Mohebbi et al., 2012), mirror carp (Xu et al., 2010) and common carp (Naeiji et al., 2013) as evidenced by decreased thiobarbutic acids in serum and tissues. Garlic has powerful antioxidant activity by scavenging reactive oxygen species (ROS) and inhibiting lipid peroxidation (Carmia, 2001). Metwally (2009) reported that antioxidant S-allyl cysteine sulfoxide which isolated from garlic controlled lipid peroxidation.
Live organisms affected by pathogens and various stress factors are exposed to oxidative stress induced free radicals, and the living organisms are threatened from these dangerous free radicals through the activities of the antioxidant system (Ritola et al., 2002). SOD and CAT enzyme levels are the most considerable indicators of these antioxidant activities (He et al., 2015). The first defense mechanism against ROS may be the induction of SOD/CAT system (Nwani et al., 2010). SOD is considered as the first line of defense against oxygen toxicity, highly sensitive antioxidant enzyme and responds more quickly thereby protecting organisms from oxidative stress due to its inhibitory effects on oxy-radical formation, catalyzed the dismutation of the superoxide anion radical into water and hydrogen peroxide after which SOD is detoxified by CAT (Rao, 2006). our study results show that, SOD activity was significantly (p≤ 0.05) increased in fish group fed diet supplemented with garlic than control group and fish group fed diet supplemented with ginger. Similarly, results of Metwally (2009) revealed that SOD enzyme activity increased in tilapia groups in which garlic used as herbal immune-stimulator. In addition, Jahanjoo et al. (2018) showed high activity of SOD in group of fish fed diets supplemented with 1% garlic or ginger. Moreover, garlic has the ability of enhancing catalase activity in serum of fish Diab et al. (2002). In the same trend, results of Diab et al. (2002) showed that, garlic used in fish farming enhanced the activity of non-specific defense systems in O. niloticus.
CONCLUSION
Generally based on the result of growth performance and body composition, supplementation of garlic or ginger in diet of Nile tilapia fish at level 1.5% did not have growth promoters’ effect. Although, supplementation with ginger and garlic in 1.5% concentration significantly decrease oxidative stress of muscle tissue through a significant reduction in MDA, a significant increase in SOD levels & a slight increase in CAT and GSH levels. In addition, garlic has more potent antioxidant effects than ginger.
ACKNOWLEDGEMENTS
We acknowledge support of both animal nutrition and physiology departments.
CONFLICT OF INTEREST
Authors declare that they have no conflict of interest.
AUTHORS CONTRIBUTION
All authors contributed equally to the manuscript.
REFERENCES






