Advances in Animal and Veterinary Sciences
Research Article
In vitro and in vivo Antibacterial Activity of Ethanolic Extract of Sweet Basil (Ocimum basilicum L.) Leaves against Escherichia coli in Experimentally Infected Rats
Orooba Mohammed Saeed Ibrahim*, Sarhan Rashid Sarhan
Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Baghdad, Iraq.
Abstract | The present study was carried out to investigate the antibacterial activity of ethanol extract of Ocimum basilicum against infectious diarrhea caused by E. coli. Two experiments were performed in this present study; the first one is in vitro antibacterial activity of Ocimum basilicum ethanolic extract. The second experiment included study in vivo antibacterial activity of the extract after inducing diarrhea with oral pathogenic E. coli in five rats groups (eight rats of each). The results of the in vitro antibacterial study showed that E. coli was more sensitive to ethanolic extract of O.B in comparison with Gentamicin and Amikacin and it was resistant to the metronidazole, but E. coli was more sensitive to Trimethoprim/Sulfamethoxazole. Two doses of O.B extract 100, 200 mg/kg. BW were used to treat this infection for fourteen days orally. The results of the in vivo study indicated that doses of 200 mg/kg BW O.B extract and 6.85 mg/kg. BW of Trimethoprim/Sulfamethoxazole were succeeded in returning the rectal E. coli count of infected rats to normal values before inducing infection in both kinds of therapy at the first week. Food intake, water intake and body weight significantly (P < 0.05) increased in comparison with others doses or groups. From the results obtained, it could be concluded that ethanolic extract of O.B leaves at dose 200 mg/kg. BW was more effective and safe in comparison with antibacterial agent and other O.B dose, this anti-diarrheal activity of O.B ethanolic extract may be due to its constituents of secondary metabolites that are responsible for the antibacterial activity with different mechanisms of action.
Keywords | Antimicrobial activity, Escherichia coli, Ocimum basilucum, Diarrhea
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 23, 2015; Revised | April 19, 2015; Accepted | April 21, 2015; Published | May 05, 2015
*Correspondence | Orooba Mohammed Saeed Ibrahim, Baghdad University, Baghdad, Iraq; Email: [email protected]
Citation | Ibrahim OMS, Sarhan SR (2015). In vitro and in vivo antibacterial activity of ethanolic extract of Sweet Basil (Ocimum basilicum L.) leaves against Escherichia coli in experimentally infected rats. Adv. Anim. Vet. Sci. 3(6): 308-320.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.6.308.320
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Ibrahim and Sarhan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Diarrheal diseases are a major problem in third world countries which are responsible for death of millions of people and animals each year, diarrhea is an alteration in normal bowel movement and it is characterized by an increase in the water content, volume, or frequency and decrease of dry matter of feces (Shoba and Thomas, 2001; Guerrant et al., 2001; Rodostitis et al., 2007). Diarrhea are associated with loss of electrolytes and fecal matter, decreased fluid absorption or increased fluid secretion can lead to dehydration, it can be either acute or chronic (Sandler et al., 2000). Diarrhea accounts of 46% of calves and lambs mortality (Schoenian, 2007). The most common causes of acute diarrhea are bacterial and viral infections (Holland, 1990; Brandt and Greenwald, 2001). Rota virus is responsible for causing severe diarrhea, which leads to gastroenteritis (Nappert et al., 2000). Bacterial causes like Escherichea coli, Staphylococcus aureus, Sallmonella and Vibrio cholera, also parasitic infection, especially protozoa can lead to severe acute diarrhea which is responsible for the high levels of mortality and morbidity in humans and animals (Holland, 1990; Huang et al., 2002). Infection with Escherichea coli being one of the major causative agents (Ansaruzzaman et al., 2000). E. coli infection occurs all over the world and affect all farm animals in all ages, it can cause white scour in newborn calves and to less extent diarrhea in lambs, kids etc. and septicemic colibacillosis in lamb, foals, calves and kids (Quinn et al., 2004). Diarrheal disease is still a major health problem, especially in developing countries, where it is considered as one of the leading causes of morbidity and mortality especially in children. Among the bacterial pathogens, diarrheagenic Escherichia coli (DEC) is one of the important etiological agents of diarrhea (Blanco et al., 2005; Moyo et al., 2007; Asadi et al., 2010). Commensal Escherichia coli, which was discovered in 1885, is the predominant facultative anaerobe of the human gut microbiota (Lai et al., 2013). Diarrheagenic E. coli (DEC) strains can be divided into six main categories on the basis of distinct epidemiological and clinical features, and specific virulence determinants (Blanco et al., 2006): Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Enteroinvasive E. coli (EIEC), Enterohaemorrhagic E. coli (EHEC) or Shiga-toxin producing E. coli (STEC), Enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC) (Lozer et al., 2013). Calf mortality has been reported to be very high in cow and buffalo neonates (Khan et al., 1991). According to a survey on mortality rate in calves vary from 2% to 20% due to diarrhea in advanced countries. Locally Al-khayyat et al. (1977), noticed after study included 100 fecal specimens collected from animals suffering diarrhea from different areas of Baghdad, E. coli was isolated from 68 specimens. The highest infection rate with E. coli was recorded by Perez et al., (1998) in Costa Rica, it’s was 94% from calves suffering diarrhea. Colibacillosis, associated with E. coli, occurs in all species of new born farms animals and it is a major cause of economic loss in this age and it cause diarrhea particularly in calves less than 30 days of age (Rodostitis et al., 2007). It is a common cause of gastroenteritis and it accounts for 30% of the total number of diarrhea pathogenic in some regions of the world (Alikhani et al., 2007). Resistance to the anti-microbial agents is recognized as a major global public health problem, infectious diseases are for approximately one–half of all cases of death in different beings (Iwu et al., 1999). For these reasons, international organizations including the WHO have encouraged studies pertaining to treatment and prevention of diarrheal diseases using traditional medical practices (Park, 2000), it is possible that antimicrobial compounds from plants may inhibit bacteria by different mechanism than the presently used antibiotics and may have a clinical value in treatment of resistant strains (Cox and Balick, 1994). Plants have long been a very important source of new drugs, many plant species have been screened for substances with therapeutic activity and for those medicinal plants are promising source of antidiarrheal drugs (Maikere-Faniyo et al., 1989). Therapeutic value of plants used in trade medicine derives from the presence of phytochemicals principles (secondary metabolites) which are found in all parts of plant such as alkaloids, tannins, flavonoid, saponins and phenols (Ayodele, 2003). One of the most important medicinal plants is Ocimum basilicum (Sweet basil), which is a seasonal plant belongs to the family of Lamiaceae. Ocimum plant is an important medicinal plant as it is a well known source of different phytochemicals, it is distributed throughout most of the world and it is abundant in Iraq. Ocimum basilicum (OB) can be used as an antioxidant, antiseptic, preservative, sedative, digestive regulator and diuretic. It is also recommended for treatment of headaches, coughs, infection of upper respiratory tract, kidney malfunction and to eliminate toxins (Evans et al., 2006). OB is also used in the treatment of a number of ailments like bronchitis, rheumatism and pyrexia (Keita et al., 2000). Also Ocimum plant rich in secondary metabolites which have antibacterial activity by different mechanisms (Durga et al., 2010). Therefore, his study was presented to evaluate the antibacterial activity of ethanolic extract of Ocimum basilicum against E. coli as well as treatment of diarrhea and avoiding resistance to antimicrobial agents. These were accomplished by studying the in-vitro antibacterial activity of plant extract and in vivo through treatment of experimentally infected rats with E. coli comparing with different antibiotics. As well as overcome the resistance to antibacterial drugs.
MATERIALS AND METHODS
Test Organism
Enteropathogenic E. coli ICC 223 (EPEC O125:H6) strain was obtained from the College of Veterinary Medicine/Department of Internal and Preventive Medicine/University of Baghdad. This isolates spp. was identified by studying morphological and some biochemical characteristics.
Plant Material
Fresh Ocimum basilicum leaves were purchased from a local market in Baghdad during May to August 2010. Later these plant leaves were washed with tap water, and then dried at room temperature at shade. The dried leaves were crushed to a fine powder by an electrical grinder. The plant classification was done in the Ministry of Agriculture/ State Board for Seeds Testing and Certification S.B.S.T.C in Abu Graib/Baghdad at certificate No. 3670 in 28 / 11 / 2010.
Preparation of Crude Organic Solvent
Extaction of O. Basilicum Plant
Organic solvent extraction of the Ocimum basilicum leaves was carried out by using ethanol (95% ethyl alcohol, BDH England) which is considered as very effective in extracting the active ingredients of the plant according to method described by Effraim et al. ( 2000). This was done by using Soxhlet apparatus (Electrothermal, England). 50g of plant leaves powder was put inside the thumble and 500 ml of 95% ethanol was put in the flask. The extraction was carried out for 24 hours by heating temperature that kept the solvent at 50-60°C until a clear and colorless solvent appeared in the extracting unit. After that, the extract was dried by using an electric oven at temperature 40-45°C until dry extract was obtained. The dry extract was placed in an incubator under 38-40oC for complete dryness of the sample. The final extract was kept frozen at –20°C until use.
Detection of Thephytochemicals Components of O. Basilicum
Chemical tests were carried out on the plant powder and its ethanol extract by using standard procedures to identify the constituents. All chemical reagent and solutions supplied by BDH, England.
Preparation of Different Concentration of Plant Extract
Stock solutions were prepared by mixing 1g of dried extract with 10 ml of 50% Dimethyl- sulphoxide DMSO (Fluka, Germany) that was sterilized with Millipore membrane filter (0.20µm). Then concentrations of 10, 20, 40, 60, 80 and 100 mg/ml were prepared by mixing known volume from the stock solution with 50% DMSO.
Antibacterial Susceptibility Test
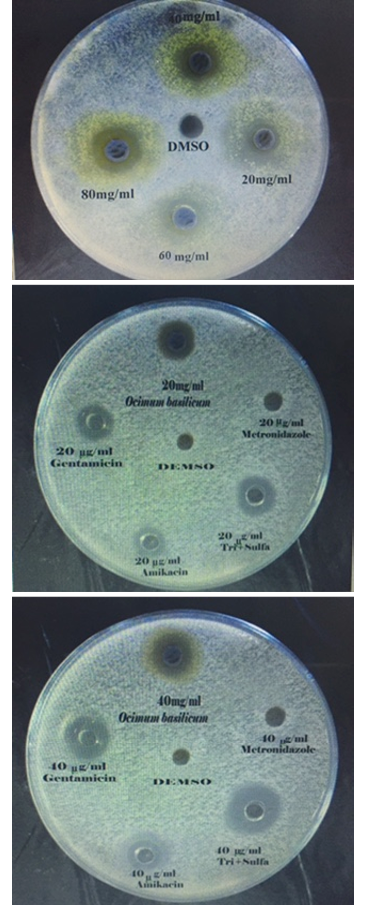
The agar well diffusion method was adopted (Grove and Randall, 1955; Bauer, 1966; Kavanagh, 1972), for assessing the antibacterial activity of the prepared extract. 5ml of standardized bacterial stock suspensions (the average number of viable Escherichia coli cell per ml of the stock suspension was determined by means of the Standard McFarland solution No.0.5. (1.5 × 108 cfu/ml) of E. coli was thoroughly mixed to 500 ml of sterile Mueller Hinton agar. 25 ml of the inoculated Mueller Hinton agar was distributed into sterile Petri dishes of each. The agar was left to set for 10 minutes to allow solidifying the agar, and in each of these plates 6 well, 6mm in diameter were cut using a sterile Pasteur pipette and the agar discs were removed by a sterile forceps, after that wells were filled with 0.1ml of each concentration of (10, 20, 40, 60, 80 and 100 mg/ml) of Ocimum basilicum extract using microtiter-pipette that allowed to diffuse at room temperature for two hours. The plates were then incubated in the (upright position) to keep the plant extract in the wells at 37oC for 24 hours. Three replicates were carried out for each concentration extract and the activity of plant extract was determined by measuring the diameter of inhibition zone around each well by millimeter against the tested organism. Simultaneously, addition of the respective solvent (50% DMSO) instead of extract was carried out as controls. The results and standard errors means values were tabulated.
In Vitro Antibacterial Activity of Standard Antibiotics
Gentamicin (Menarini international, Italy) Amikacin (Kontam, India), Metronidazol (Mission Vivacare, india) and Trimethoprime /Sulfamethoxazole (NDI, Iraq) were used as a reference antibiotics to determine sensitivity of E. coli tested (Sharma and Patel, 2009). The same technique which was used for antibacterial sensitivity of extract was used for determination of Standard antibiotic activity by using the concentrations of 10, 20, 40, 60, 80 and 100 mcg/ml., 0.1 ml of sterilized distilled water was served as a control.
Experimental Animals
Forty eight male Wister albino rats about three months of age and with body weight ranged between 190-210g were used to perform the experiment of the present study. Rats were housed in plastic cages 20×50×75cm dimension, placed in a special housing room belongs to the Department of Physiology and Pharmacology / College of Veterinary Medicine for two weeks for adaptation. Standard rodent diet (Commercial feed pellets) and tap water were freely available. Housing condition were maintained at 20-25oC in air-conditioned room, the air of the room was changed continuously by using ventilation vacuum, while the light/dark cycle was 14/10 in housing place. The litter of the cages was changed weekly. The protocol was approved by the Animal Care and Use Committee of the University of Baghdad, College of Veterinary medicine prior to the initiation of the study.
In Vivo Antibacterial Activity of Ethanol Extract of O. Absilicum on Rats Antibiotics
Challenge Bacteria
Enteropathogenic E. coli ICC 223 (EPEC O125:H6) strain was obtained by Department of Internal and Preventive Medicine in College of Veterinary Medicine/Baghdad university from a calve suffering from diarrhea; it was used as a challenge strain.
Inducing Infection (Diarrhea)
After pilot study, the challenge dose which induced infection (acute diarrhea) was 2.5 x 106 cfu/ml of E. coli suspension, the inoculums preparation-standardize according to viable counting method-pour plate technique by using serial ten-fold dilutions. 0.5 ml was orally administered to the rats and watched for symptom of diarrhea. The dilution that established the infection in the rat which showed by the symptoms was used as infectivity dosage for the rats throughout the infection (Bitton, 2005; Quinn et al., 2004). Overnight culture (24 h at 37oC) in brain heart infusion broth dilution of 0.5ml was given with a gavages stomach tube to each rat of infected groups.
Preparation of Different Concentration of Plant Extract for In Vivo Study
Stock solutions were prepared by mixing 1g, 2g from dried extract with 10 ml of 50% DMSO separately, it was filtered through whatman (No.1). to prepare the concentrations of 100 and 200 mg/ml respectively. These concentrations were used for daily dosing of treated groups.
Experimental Design
Forty eight rats were divided equally into six groups, eight rats in each group (Treatment begin after 24hrs. after inducing infection). Group(A): positive control (infected and not treated group), Group(B): negative control (not infected group which given only 50% DMSO orally for 14 days, Group(C): infected and treated orally with 100 mg/kg B.W of ethanolic extract of Ocimum basilicum (OB) for 14 days, Group(D): infected and treated orally with 200 mg/kg B.W of ethanolic extract of (OB) for 14 days, Group(E): infected and treated orally with 3.57 mg/kg B.W of Metronidazol at concentration 0.7mg/ml for 14 days, Group (F): infected and treated orally with 6.85 mg/kg B.W of Trimethoprime /Sulfamethoxazole at concentration 1.35mg/ml for 14 days. The experimental Durations were as follows: One week before inducing infection. Two weeks of treatment after 24hr inducing infection.
Clinical Signs
Clinical signs were checked continuously for color, consistency of feces and development of diarrhea in infected groups, also any change in activity, behavior were recorded weekly throughout the experiment.
Body Weight Change
Weight of the animals was measured and compared with food consumption of treated animals against the control. These measurements were done weekly for three weeks; First week-before inducing infection, after 7 days of treatment and after 14 days of treatment. This was done in order to know the effect of infection and the possible effect of the extract and antibacterial agent on body weight.
Water Consumption
Water intake was measured weekly (for three weeks of the experiment), in order to know the quantity of drinking water, and effect of infection and treatment in animals water consumption.
Food Intake
Food intake was measured weekly (for three weeks of the experiment), in order to know the quantity of food intake by the animals and the effect of infection and treatment in food intake.
Rectal Bactrial Count of E. Coli
Fecal specimens were obtained with rectal sterile cotton swabs. E. coli bacterial counts in fecal samples were performed before infection, a day after infection and at the end of 7 and 14 days of treatment. Fecal samples in about 100 mg were suspended in 1 ml of diluents containing 0.1% peptone in 0.85% saline in a ratio of 1:99. The initial nutrient broth suspensions and their tenfold serial dilution were cultured (0.1 ml) in duplicate on MacConkey Agar (according to pour plate method for bacterial count). Typical smooth pink color colonies were counted after incubation for 24 hours at 37˚C. Plates inoculated with a sample dilution that yields between 30-300 colonies per plate were read (Sarelli et al., 2003; Oyetayo et al., 2003). The number of bacteria (cfu) per ml or gram of sample was calculated by dividing the number of colonies by the dilution factor multiplied by the amount of specimen added to liquefied agar.
Number of colonies (CFUs) = number of bacteria (ml)/dilution × amount plated (Quinn et al., 2004)
All media mentioned above are supplied by Himedia, India.
Statistical Analysis
Data were analyzed statistically using the Microsoft Program (SPSS). Statistical analysis of data was performed on the basis of Two-Way Analysis of Variance (ANOVA) using a significant level of (P<0.05). Specific group differences were determined using least significant differences (LSD) as described by (Snedecor and Cochran, 1973).
RESULTS AND DISCUTIONS
Extraction of Ocimim Basilicum
Extraction of plant leaves with 95% ethanol gave a deep green color extract with plant powder yield percentage of 13% , this was determined by using the following equation: Percentage yield of the extract = weight of extract (gm) / weight of Ocimum powder (gm) × 100 =13(gm) / 100(gm) × 100 = 13% (Banso and Adeyemo, 2006). This result is almost similar to the results of Tomar et al. (2010) who found that the percentage recovery of ethanolic extract was 10.6% w/w from fine ocimum leaves powder which was extracted by using a soxhlet apparatus. The near similarity in yield percentage may be attributed to the same solvent which was used in the present extraction.
Detection of Thephytochemicals Components of O. Basilicum Leaves Powder and its Extract
Active components in Ocimum basilicum plant leaves powder (before extraction), and in its 95% ethanolic crude extract and results of detection were listed in (Table 1). The plant leaves powder screening showed the presence of the following phytochemicals; alkaloids, phenol, saponins, flavonoids, tannins and glycosides, while steroids, terpenoids did not exist in plant powder. The detection of phytochemicals in ethanolic extract gave an evidence of the existence of the following components; alkaloid, phenols, steroids, saponins, flavonoids, tannins and terpenoids while glycosides were absent. Alkaloid, phenols, saponins and tannins seemed to be found in a high level in the crude extract. Cowan (1999) referred that the initial screenings of plants for possible antimicrobial activities typically begin by using crude aqueous or alcoholic extractions and it can be followed by various organic extraction methods. Since nearly all of the identified components of the plant which are known by their activity against microorganisms are aromatic or saturated organic compounds, they are most often obtained through ethanol or methanol extraction. The
Table 1: Phytochemical analysis of Ocimum basilicum leaves
|
Test |
Alkaloids |
Flavonoids |
Phenols |
Saponins |
Tannins |
Terpenoids |
Steroids |
glycosides |
||
|
Mayer’s |
Dragen * |
Sterring |
Lead acetate |
Ferric chlorides |
Fehling T. |
|||||
|
O.B leaves powder |
++ |
++ |
+ |
++ |
++ |
+ |
+ |
- |
- |
+ |
|
O.B 95% ethanolic extract |
+++ |
+++ |
+ |
++ |
+++ |
++ |
++ |
+ |
+ |
- |
|
Remarks |
White prec.** |
Orange prec. |
Yellow color |
Blue-dark green |
Big foam |
Gelatin-white color |
Blue-green color |
Brown color |
Blue-green color |
Red prec. |
+ = positive; ++ = good present; +++= strongly present; – = not detected; * = dragendroff; **= precipitate
Table 2: In vitro antibacterial activity of O.B extract in different concentrations on E. coli (diameter of inhibition zone in mm)
|
Concentration mg/ml |
||||||
|
Zone of inhibition mm. |
100 |
80 |
60 |
40 |
20 |
10 |
|
Ethanol extract of O.B |
23.0±0.61 A a |
22.0±0.91 A a |
17.0±0.73 B a |
15.0±0.38 C a |
12.0±0.77 D a |
09.0±0.36 E a |
|
50% DMSO |
0.0±0.0 A b |
0.0±0.0 A b |
0.0±0.0 A b |
0.0±0.0 A b |
0.0±0.0 A b |
0.0±0.0 A b |
Values represent mean ±S.E; Different capital letters mean significant (P<0.05) results among different concentrations. Different small letters mean significant (P< 0.05) results between solvent and Extract.
ethanol extract of O. basilicum was more effective against the pathogens when compared with the acetone extract as confirmed by Durga et al., (2010), it all about using the right solvent for extraction of these phytochemicals that have antimicrobial activity. Crude products obtained from alcoholic extraction can then be used in disc diffusion and broth dilution assays to test for antifungal and antibacterial properties and in a variety of assays to screen for antiviral activity (Cowan, 1999).
Identification of E. Coli
The strain had a smooth circular pink colony on MacConkey agar and displayed wide hemolytic zones around the colonies on Blood agar and a metallic green sheen on Eosin Methylene Blue agar. The challenge bacteria were the same strain which was used in in-vitro antibacterial activity assay.
In Vivo Antibacterial Activity of Ethanol Extract of O. Absilicum Leaves
Different concentrations of ethanolic extract of Ocimum were used in agar well diffusion assay, caused different degrees of zones of inhibition against E. coli. The size of inhibition zones were different according to the concentration of the extract, the size of inhibition zone were proportionally increased with increasing of the oncentration of ethanolic extract (Table 2 and Figure 1). The results showed that E. coli were more sensitive to ethanolic extract of Ocimum than Amikacin and Gentamicin in all the concentrations using in this study except in concentration 10 mcg/ml in case of Gentamicin. In all used concentration there was a significant increase (P<0.05) in diameter of the zone inhibition of E. coli growth when it compared with zone of inhibition of Amikacin and Gentamicin. The E. coli was more sensitive to Trimethoprim/ Sulfamethoxazole while it was resistant to Metronidazol. Dimethylsulfoxide (DMSO) 50% was used as control, it did not give any noticed zone of inhibition, 50% DMSO was used as a solvent for Ocimum basilicum crude extract in both in-vitro and in vivo studies, it is considered as one of the solvents that can be used for screening the antimicrobial activity of plant extracts because of its 100% biologically inert substances (Fardos, 2009). The results of inhibitory zone diameter indicated the sensitivity of E. coli after 24hrs of incubation, towards different tested extract concentrations. All antibacterial activities were observed to be concentration dependent that is in agreement with Tomar et al., (2010) who attributed the antibacterial activity of Ocimum to its composition of bioactive secondary metabolites that produced definite pharmacological and physiological action, some of the most importants include; alkaloid, flavonoids, phenol, tannins, saponins, steroids and terpenoids which have antibacterial activity with different mechanisms of action (Krishnaiah et al., 2009). Many medical plants exert their beneficial effect through the additive or synergistic action of several chemical compounds acting at
Table 3: Antibacterial activity of the reference antibiotics used against E. coli
|
Concentration mg/ml |
Antibiotics |
||||
|
Zone of growth inhibition (mm) |
|||||
|
Metronidazol |
Amikacin |
Gentamicin |
Trim/Sulfa |
DW |
|
|
100 |
0.0±0.0 A d |
15.0±0.27 A c |
19.0±0.32 A b |
32.0±0.43 A a |
0.0±0.00 A d |
|
80 |
0.0±0.0 A d |
13.0±0.57 B c |
17.0±0.14 B b |
27.0±0.28 B a |
0.0±0.00 A d |
|
60 |
0.0±0.0 A d |
11.0±0.12 C c |
14.0±.33 C b |
24.0±0.70 C a |
0.0±0.00 A d |
|
40 |
0.0±0.0 A d |
10.0±0.14 D c |
12.0±0.51 D b |
21.0±0.91 D a |
0.0±0.00 A d |
|
20 |
0.0±0.0 A d |
09.0±0.34 E c |
11.0±0.49 E b |
18.0±0.73 E a |
0.0±0.00 A d |
|
10 |
0.0±0.0 A d |
07.0±0.00 F c |
10.0±0.37 F b |
12.0±1.02 F a |
0.0±0.00 A d |
Values represent mean ±S.E; Different capital letters mean significant (P<0.05) results among different concentrations; Different small letters mean significant (P< 0.05) results among different antibiotic.
single or multiple target sites associated with physiological processes as pointed by Tyler (1999), that may explain the difference in diameter of inhibition zone between the extract and the antibiotics. The results are in agreement with Nwinyi, (2009) who referred that ethanol extracts exhibited high inhibitory activity on the test organisms; this can be deduced to the ability of ethanol to extract more of the essential oils and secondary plant metabolites which are believed to exert antibacterial activity on test organisms. So this may explains the difference in inhibition zone diameter against E. coli.
In Vitro Antibacterial Activity Of Standard Antibiotics
Gentamicin, Amikacin, Metronidazole and Trimethoprime/Sulfamethoxazole were used as reference antibiotics, E. coli was sensitive signific-antly (P<0.05) to Trimethoprime /sulfamethoxazole more than Gentamicin and Amikacin in a dose dependent concentration 100, 80, 60, 40, 20, and 10 mcg /ml (Table 3 and Figure 1). Sulfonamide blocks the conversion of para- aminobenzoic acid to dihydrofolic acid, and trimethoprim blocks the conversion of dihydrofolic acid to tetrahydrofolic acid by inhibition of dihydrofolate reductase. This gave a synergistic effect for Trimethoprim /sulfamethoxazole (Plumb, 2004). While E. coli was resistant to Metronidazole, evidence obtained from laboratory and epidemiological studies indicated that the persistence of resistant bacteria was related to the persistence of antimicrobial drug use (Andersson, 2003). If an antimicrobial drug is used, continuously, the persistence of resistant organisms will go on. Thus, E. coli has often higher degrees of antimicrobials resistance which have a long history of use (Alhaj et al., 2007). Series of studies on the resistance of E. coli which were isolated from animals and humans strongly suggested that those bacteria which are resistant to antimicrobials used in animals would also be resistant to antimicrobials used in humans (WSPA, 2006; Miles et al., 2006; Umolu et al., 2006).
Physiological Changes
Clinical Signs
Before induction of infection, healthy animals presented normal feces; sold molded, rough with dark brown color figures, while after inducing infection animals, were suffering from anorexia, dehydration, little fever or no fever and the feces was unmolded-wet lose or liquid appearing and light brown in color. The frequency of diarrheal feces increased gradually from the first day after infection. All the animals exhibited clinical signs of abnormal behavior like; calmness, less mobile and curled up. The animals of group C and E which received 100 or 3.57 mg/kg B.W of ethanolic extract of Ocimum basilicum and metronidazol, respectively for 7 days did not show complete recovery at the end of 7 days of treatment and clinical signs were relatively mild until 14 days of treatment the signs began subside, while the animals of group D which dosed with 200 mg/kg .B.W of ethanolic extract of O.B and group F which dosed with 6.85mg/kg B.W of Trimetoprim /Sulfamethoxazole for 7 days exhibited faster recovery. Group A (+ve control) untreated group showed; severe diarrhea, dyspnea, emaciation, rough body coat, poor body weight gain and reduced elasticity of the skin indicating dehydration. Morbidity (infection) rate was 100% in infected groups, the highest recorded mortality rate was 75% in group A (+ve control) during the period of experiment while groups C and E (treated groups) showed fluctuating rates of mortality, groups D and F did not lose any animals during the period of experiment. Forrester, (2002) reported in rat that infectious diarrhea with E. coli gave clinical signs developed in steps began with production of thin and watery feces, fever, loss of appetite, signs of dehydration appear (sunken eyes, dry mucus membranes, rough hair), unable to rise body, loss of consciousness, dehydration and death can be less than 24 hours.
Food Intake
Induce infection in different groups lead to fluctuated changes in food intake. The infection was negatively proportional with O.B ethanolic extract treatment doses in their effect on decreasing animal’s body weight. The effects of infection and treatment on food intake (rat-gm /week) are listed in (Table 4). There was a significant decrease (P< 0.05) in food intake in all infected groups after inducing infection in comparison with group B (–ve control). These results are in agreement with (Rout et al., 2002), who confirmed the decrease in food intake in rats after infection with E. coli. After two weeks of treatment, there was a significant decrease (P< 0.05) in all infected groups with different levels, but group A (+ve control) kept its decrease of food intake in comparison with group B (–ve control) at the same period of the experiment. Groups C, D and F showed a significant decrease(P< 0.05) positively proportional with their therapeutic doses of extract and Trimethoprim /Sulfamethoxazole. The decrease in food intake in groups D, and F was in lower values in comparison with other ethanolic extract of O.B dose and Group E which treated with Metronidazol.
Table 4: Food intake (rat-gm/week) values of rats in different groups infected and treated with different doses of ethanolic extract of O.B and Metronidazol, Trimethoprim/Sulfamethoxazole or kept without treatment during course of experiment
|
Period |
||||
|
After 14 days of treatment |
After 7 days of treatment |
After 24 hr inducing infection |
Week before infection |
Group |
|
65±1.82 D c |
48±0.80 D d |
77±1.49 B b |
82±1.68 A a |
G A +ve control |
|
100±0.7 A a |
99±0.31 A a |
89±0.39 A b |
82±0.36 A c |
G B –ve control |
|
89±0.22 B a |
80±0.92 B c |
72±1.46 B d |
83±0.44 A b |
GC: O.B extract 100mg/kg |
|
90±0.33 B a |
83±1.13 B b |
76±0.55 B c |
83±0.29 A b |
G D: O.B extract 200mg/kg |
|
76±0.81 C b |
66±0.74 C c |
77±0.91 B b |
80±0.73 A a |
G E:Metronidazol 3.57mg/kg |
|
93±0.41 B a |
90±.80 B b |
74±1.00 B d |
83±1.09 A c |
G F: Trim/Sulfa. 6.85 mg/kg |
Values represent mean ±S.E, Group rat no.= 8; Different small letters mean significant (P<0.05) results among periods; Different capital letters mean significant (P< 0.05) results among groups; +ve control: infected not treated group; − ve control: not infected, not treated group.
Water Consumption
Inducing infection in different groups lead to fluctuated changes in rats water intake, the same negative interaction between the infection and whether receiving treatment or not, kind of treatment and their dose were noticed as reported in food intake. The effect of infection and treatment on rat water intake (rat-ml/week) listed in (Table 5). The results showed a significant increase (P<0.05) in water intake during week of inducing infection and treatment in all five infected groups in comparison with group B (–ve control) at the same period of the experiment, the highest level was recorded in group A (+ve control). A little decrease of water intake was noticed between different groups after 7 days of treatment, group A also showed the highest weekly water intake when it was compared with the control group B (–ve control), while treated groups after 14 days of treatment had less significant values and they began to return to normal water intake values.
Table 5: Water intake (rat-ml/week) values of rats in different groups infected and treated with different doses of ethanolic extract of O.B and Metronidazol, Trimethoprim/Sulfamethoxazole or kept without treatment during the course of experiment
|
Period |
||||
|
After 14 days of treatment |
After 7 days of treatment |
After 24 hrs. inducing infection |
Week before infection |
Group |
|
220±0.16 B c |
245±0.80 A a |
230±0.34 A b |
210±1.09 A d |
G A +ve control |
|
212±0.72 D a |
215±0.23 C a |
215±0.77 B a |
210±0.82 A a |
G B –ve control |
|
215±1.10 C c |
221±0.62 B a |
225±1.20 A a |
211±0.53 A c |
Gc: O.B extract 100mg/kg |
|
211±0.29 D c |
216±0.82 C a |
220±0.76 A a |
211±0.67 A c |
G D: O.B extract 200mg/kg |
|
224±0.40 A b |
236±1.02 A a |
222±0.91 A b |
210±0.60 A c |
G E:Metronidazol 3.5mg/kg |
|
214±0.52 C b |
210±0.53 D c |
220±0.60 A a |
211±0.89 A b |
G F: Trim/Sulfa. 6.85 mg/kg |
Values represent mean ±S.E, Group rat no.= 8; Different small letters mean significant (P< 0.05) results among periods; Different capital letters mean significant (P< 0.05) results among groups; +ve control: infected not treated group; −ve control: not infected, not treated group.
Body Weight Change
The changes in body weight (rat-gm/week) gave an evidence of correlation between infection, the kind and dose of treatment that used in different groups, as in (Table 6). After 24hs of inducing infection, there was a significant decrease (P<0.05) in body weight in groups A ,C, D, E and F when they were compared with group B (–ve control), These results are in agreement with Ogundare and Onifade (2009) who reported a decrease in body weigh after infection with E. coli. After 7 days of treatment, animals of groups D and F showed faster weight gain, while the other treated groups showed a little weight gain in comparison with group B (–ve control). The same pattern was noticed at the second week of treatment in which group A persisted in its significant decreasing in comparison with the control group B (–ve control) and also when it was compared with the other groups. Group F did not show a significant difference in comparison with its weight at the first week of treatment, while group D continued in its normal significant increase of weight gain throughout the experimental period. That explained the highly potency of antibacterial compounds of ethanol extract of O.B, the same thing was with synergetic effect of Trimothoprim /Sulfamethoxazole. Infection induced strong anorexia that was associated with a loss of body weight in all infected groups. Thereafter, body weights began to recover as food intake increased progressively, to reach values similar to those observed before infection at the end of treatment (day 14). Infected rats of group A lost more body weight. These results were confirmed by (Rout et al., 2002). However E. coli endotoxin and interlukin-1(IL-1) will increase the renal excretion of arginine vasopressine. It is possible that injection of bacterial endotoxin or IL-1 will induce changes in fluid intake that would be reflected as an increase intake of liquid and water (McCarthy et al., 1985). The increase of water intake in infected groups may belong to the increase of body temperature and diarrhea which lead to loss fluids and dehydration. Loss of food appetite is a common manifestation of acute infectious illness and it is believed to contribute to the negative nitrogen balance and loss of body weight that is seen during infection. This
Table 6: Weight change (rat-gm/week) values of rat in different groups infected and treated with different doses of ethanolic extract of O.B and Metronidazol, Trimethoprim/Sulfamethoxazole or kept without treatment during the course of experiment
|
Period |
||||
|
After 14 days of treatment |
After 7 days of treatment |
After 24 hrs. inducing infection |
Week before infection |
Group |
|
160±0.68 E d |
170±0.67 E c |
184±0.45 B b |
196±0.23 A a |
G A +ve control |
|
207±0.61 A a |
203±1.12 A b |
194±0.73 A c |
195±1.01 A c |
G B –ve control |
|
192±0.81 C b |
188±0.77 D c |
185±0.53 B d |
195±0.59 A a |
G C: O.B extract 100mg/kg |
|
196±0.56 B a |
192±0.91 C b |
183±0.34 B c |
195±0.50 A a |
G D: O.B extract 200mg/kg |
|
188±1.00 D b |
186±0.96 D c |
184±0.40 B c |
195±0.42 A a |
G E:Metronidazol 3.5mg/kg |
|
195±0.32 B a |
198±0.61 C a |
186±1.06 B b |
197±0.56 A a |
G F: Trim/Sulfa. 6.85 mg/kg |
Values represent mean ±S.E, Group rat no.= 8; Different small letters mean significant (P< 0.05) results among periods; Different capital letters mean significant (P< 0.05) results among groups; +ve control: infected not treated group; −ve control: not infected, not treated group.
hypothesis tested by injection of E. coli endotoxin into rat which resulted in both an elavation in body temperature and depression in food intake (McCarthy et al., 1985).
Rectal Bacterial Count
Rectal bacterial count was done according to pour plate method. Grabow et al. (1992) compared with many methods to enumerate fecal E .coli and found that this method and spread plate method on MacConky agar are easier and more convenient to be carried out, it is less labor intensive and less expensive, and gives results for fecal E. coli that are available within one day.
Table 7: Rectal bacterial count of E. coli (log) in different groups infected and treated with different doses of ethanolic extract of O B and Metronidazol, Trimethoprim/Sulfamethoxazole or kept without treatment during the of experiment
|
Period |
||||
|
After 14 days of treatment |
After 7 days of treatment |
After 24 hrs. inducing infection |
Week before infection |
Groups |
|
5.87±32.6 A b |
6.85±29.1 B a |
9.27±33.3 A a |
2.79±20.3 A c |
G A +ve control |
|
2.77±33.7 C a |
2.77±23.3 A a |
2.79±35.6 B a |
2.82±21.4 A a |
G B –ve control |
|
2.85±30.3 C a |
4.80±26.9 C b |
9.23±30.3 A a |
2.83 ±25.2 A c |
GC: O.B extract 100mg/kg |
|
2.74±31.4 C b |
2.89±22.1 A b |
9.25±38.0 A a |
2.81±20.0 A b |
G D: O.B extract 200mg/kg |
|
3.85±35.9 B c |
4.83±25.2 C b |
9.20±32.9 A a |
2.85±23.4 A d |
G E:Metronidazol 3.5mg/kg |
|
2.72±37.0 C b |
2.79±22.5 A b |
9.25±31.3 A a |
2.84±19.2 A b |
G F:Trim/Sulfa.6.8mg/kg |
Values represent mean ±S.E, Group rat no.= 8; Different small letters mean significant (P< 0.05) results among periods; Different capital letters mean significant (P< 0.05) results among groups; +ve control: infected not treated group; −ve control: not infected, not treated group.
Count Before Inducing Infection
The count of E. coli bacteria for the six groups was with colony forming unit/ml (cfu/ml) for all groups, as showed in (Table 7). There were no significant differences (P<0.05) between the six groups. These results of bacterial count of E. coli in normal flora are in agreement with (Pelan-Mattocks et al., 2000; Sarelli et al., 2003). E. coli occurs as a part of the normal flora in the lower part of the intestine of warm-blooded animals (Harley, 2002). Fecal coliforms include genera that originate in feces like E. coli (Doyle and Erickson, 2006). Results of other studies showed that anaerobic bacteria outnumber aerobic bacteria by a factor of 100-1000. Whereas aerobes (facultative anaerobes) such as E. coli, Enterobacter, Enterococcus, Klebsiella, Lactobacillu and Proteus are among the subdominant genera, every being has several hundreds of species belonging to these genera. The species are vary greatly in their facultative anaerobes, but composition pattern usually remains constant even in some instances like; acute diarrheal illnesses, antibiotic treatment, or to lesser extent induced by dietary interventions (Guarner and Malagelada, 2003). Narins (2003) indicated that the lower regions of the small intestine contain more bacteria and a wider variety of species, including coliform bacteria such as E. coli, so that probably the most principle causes of low viable bacterial count of E. coli in distal region of colon were regarding to the fact that the lower small intestine, caecum and right colon, fermentation is very intense with the high production of short-chain fatty acids, an acidic pH (5–6), and rapid bacterial growth. In contrast, the substrate in the left or distal colon is less available, the pH is close to neutral, putrefactive processes become quantitatively more important, and bacterial populations are close to static (Fallingborg, 1999).
Count after Inducing Infection
The second step of E. coli fecal count was done after 24 hrs of inducing infection (appearance of diarrhea). Results showed a significant increase (P<0.05) in E. coli viable count in all infected groups, the count increased significantly (P<0.05) in five challenged groups in the same time when they were compared with group B (–ve control), also there was a significant increase in bacterial count of infected groups when they were compared with their bacterial count before inducing infection, except group B did not show a significant change in its bacterial count when it was compared with its count before inducing infection. There was no significant differences (P<0.05) between infected groups after 24 hrs of inducing infection (Table 7). These results are in agreement with (Newsome et al., 1987; Gunzer et al., 2002) who showed the rectal bacterial count after inducing infection with pathogenic E. coli. Also these results are in agreement with (Mushtaq et al., 2005) who showed that the inoculated rats with 2.6×106 cfu/ml led to efficient colonization of E. coli within 24 hrs, after inducing experimental infection by ingestion of pathogenic E. coli. These results of the present study are in disagreement with Pluschke et al., (1983) who tested the ability of E. coli strains to colonize in the gut and to cause bacteremia in healthy new born rats. Rats were fed bacterial suspension (4×102 bacteria) in droplets from a plastic pipette tip, colonization happened after 3 or 4 days after counting by using anal swabs, to make sure that challenge dose was induced infection. Infection can be defined as equivalent to fecal shedding at moment in time, fecal shedding of (≥1000 cfu/ml) on 1-2 days after inducing infection is considered as a criterion of infection (active multiplication of pathogen in gastrointestinal tract) (Havelaar et al., 2000).
Count after 7 Days of Treatment
The results showed that treatment with both O.B extract and Trimethoprim /Sulfamethoxazole led to lower the fecal bacteria count but in different proportions especially between groups treated with two doses of O. B extract. There was a significant decrease (P<0.05) in rectal bacterial count of all treated groups in comparison with its count after inducing infection. Groups D and F showed the lowest count and they returned to normal bacterial count as before inducing infection through 7 days of treatment, while group A continued in its increasing in bacterial count. Groups C and E also showed a decrease (P<0.05) in its bacterial count, but in lower values in comparison with groups D and E. Group B (–ve control) did not show any significant difference along experiment periods (Table 7). These results gave a good evidence about the suitable therapeutic dose of plant extract that can be used as an antibacterial agent against E. coli. These findings are supported by the results of in-vitro study which referred to a high potency of different ethanolic extract concentrations of Ocimum basilicum plant against E .coli. The medicinal value of these plants depends on bioactive phytochemical constituents that produce definite physiological action. They attributed the antibacterial activity of Ocimum due to its composition of bioactive secondary metabolites that produced a definite pharmacological and physiological action, some of the most important include; alkaloid, flavonoids, phenol, tannins, saponins, steroids and terpenoids. Alkaloid antibacterial activity may be due to its ability to react with amino, carboxyl, sulfhydryl and hydroxyl groups in bacterial protein as well as nucleic acids, it is a highly reactive chemical compound that combines with proteins to give intermolecular cross-links and intercalate with DNA (Oonmetta-aree, 2005). Phenolic compound is mostly hydrophobic, it has a hydroxyl group (-OH). The importance of this group in antimicrobial activity is well known, the site(s) and number of hydroxyl groups on the phenol group are thought to be related to their relative activity to microorganisms (Cowan, 1999). The high activity of the phenolic components may be further explained in terms of the alkyl substitution into the phenolic nucleus, which is known to enhance the antimicrobial activity of phenols. It was suggested that plant products act via two main mechanisms of action; the first is related to the general hydrophobicity of plant products, which facilitates their adhesion to the bacterial surface inducing un-stabilization (Jongbloed et al., 2007). The second mechanism is the inactivation of different molecules of the bacteria such as enzymes or receptors by their adhesion to specific sites (Sharon and Ofek, 1986). Many researchers prefer the use of plant extract instead of antibiotics was due to attenuation of pathogens virulence by plant extract as opposed to the direct killing of pathogenic bacteria with antibiotic as a strategy to combat infections is an interesting concept. The idea that anti-pathogenic molecules that prevents for instance the production of toxins or abolish the ability of bacteria to adapt to the host environment would give a competitive advantage to the host immune system to allow clearance of the infectious organism (Bjarnsholt and Givskov, 2007; Gonzalez-Lamothe et al., 2009).
Count after 14 Days of Treatment
The results showed that treatment with O.B extract at 200mg/kg and Trimethoprim /Sulfamethoxazole led to lowering of fecal bacteria count before beginning the second week of treatment, so no significant decrease comparing with the first week of treatment. While animals treated with 100mg/kg extract and Metronidazol was shown a significant decrease (P<0.05) in rectal bacterial count after 14 days of treatment in comparison to its count after seven days and after inducing infection. All treated groups returned to normal bacterial count as before inducing infection through 14 days of treatment, while group A showed a little decrease (P<0.05) in bacterial count, (Table 7), this is probably the self-limiting of bacteria and body defense, The illness is typically abrupt, but can vary from mild, brief, and self-limiting to a severe disease similar to that seen in Vibrio cholerae infection (Levine, 1987). Trimethoprim and sulfamethoxazole act on different target sites, so that gave them a synergistic effect in killing the bacterial cell (Plumb, 2004). Treated groups C and E showed a decrease (P<0.05) in rectal bacterial count after 14 days of treatment but with 16%, 33% mortality rate, respectively, while treated group D and F showed a significant decrease (P<0.05) in rectal bacterial count after 7 days of treatment without any mortality reported.
Conclusion
In vitro study of antibacterial activity of O.B extract indicated that E. coli was sensitive to extract, MIC of the extract against E. coli were 0.312 mg/ml also the study gave an evidence that the tested isolate of E. coli is also sensitive to Trimethoprim/sulfamethoxazole, while resistant to Metronidazole. In vivo study showed that O.B extract at a dose of 100 mg/kg B.W has a limited antibacterial activity in comparison with 200 mg/kg B.W while O.B extract at dose 200 mg/kg has nearly the same effective as antibacterial agent (Trimethoprim /sulfa). this anti-diarrheal activity of O.B ethanolic extract may be due to its constituents of secondary metabolites that are responsible for the antibacterial activity with different mechanisms of action.
Acknowledge
Authors acknowledge to the Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Baghdad, Iraq and the department of Internal Medicine for providing the bacterial strain and guidance to the present work.
Reference