Advances in Animal and Veterinary Sciences
Research Article
Role of Rhipicephalus Annulatus Tick in Transmission of Lumpy Skin Disease Virus in Naturally Infected Cattle in Egypt
Sherin Reda Rouby1, Khaled H. Hussein1, Shawky M. Aboelhadid2*, Ahmed M. El- Sherif1
1Department of Veterinary Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Department of Parasitology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt.
Abstract | Lumpy skin disease (LSD) is an infectious viral disease of cattle that results in serious economic and international trade consequences. Recently, hard ticks have been incriminated in lumpy skin disease virus (LSDV) transmission. The present study attempted to investigate the role of Rhipicephalus annulatus ticks, collected from naturally infected animals, in the transmission of LSDV. A total of 300 naturally infected cattle exhibiting the acute signs of LSD were clinically examined. Skin nodules and R. annulatus stages were collected from the diseased cattle. Samples were examined by PCR for the P32 gene and positive samples were confirmed by direct gene sequencing. . Female engorged ticks were incubated for eggs deposition. LSDV infections in eggs and larvae that hatched from these eggs were screened by virus isolation on the chorioallontoic membrane (CAM) of embryonated chicken egg (ECE) and confirmed by PCR. Characteristic pock lesions were observed on the inoculated CAM. Positive PCR results were obtained from skin lesions, eggs, larvae and pock lesions of the CAM. Sequence analysis of the terminal 174bp of the P32 gene of LSDV sequences revealed a high degree of similarity to LSDV sequences available in Genbank database. The obtained results are highly suggestive for the possibility of the vertical transmission of LSDV in Rhipicephalus annulatus female ticks.
Keywords | Lumpy skin disease virus, Rhipicephalus annulatus, Transmission, PCR.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 26, 2017; Accepted | April 14, 2017; Published | April 29, 2017
*Correspondence | Sherin Reda Rouby, Department of Veterinary Medicine, Faculty of Veterinary Medicine,Beni-Suef University, Beni-Suef 62511, Egypt; Email: [email protected]
Citation | Rouby SR, Hussein KH, Aboelhadid SM, E Sherif AM (2017). Role of rhipicephalus annulatus tick in transmission of lumpy skin disease virus in naturally infected cattle in Egypt. Adv. Anim. Vet. Sci. 5(5): 185-191.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.5.185.191
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Rouby et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lumpy skin disease (LSD) is an acute viral cattle disease characterized by fever, nodules on the skin, mucous membranes and internal organs, emaciation, edema of the skin and enlarged lymph nodes. The disease causes significant economic losses due to hide damage, reduced milk yield and infertility in addition to the restriction of international trade of live animals and their products (Davies, 1991; Coetzer, 2004; Babiuk et al., 2008).
Lumpy skin disease virus (LSDV) belongs to the family Poxviridae, genus Capripoxvirus, of which the Neethling strain is the prototype (Buller et al., 2005). LSDV is closely related antigenically and genetically to sheeppox and goatpox viruses in the genus Capripoxvirus (Gershon and Black, 1988; Kitching et al., 1989).
Epidemics of LSDV are associated with conditions that favor high activity of biting flies especially during the wet and warm season (Kitching and Mellor, 1986; Chihota et al., 2001; Hunter and Wallace, 2001). The occurrence of LSD outbreaks has been linked with high densities of Culex mirificus and Aedimorphus natronius mosquitoes (Macowan, 1959). It was also attributed to Stomoxy scalcitrans flies (Yeruham et al., 1995). Moreover, LSDV was detected in Aedes aegypti six days after blood-feeding on infected animals (Chihota et al., 2001). Accordingly, it is widely agreed that the principle method of transmission of LSDV is mechanical by blood-feeding vectors (Kitchingand Mellor, 1986; Carn and Kitching, 1995; Chihota et al., 2001). Currently, other modes of transmission including insemination by infected semen (Annandale et al., 2014) and intrauterine transmission (Rouby and AboulSoud, 2016) have been recorded.
Recent studies shed light on the role of ticks in the transmission of LSDV (Tuppurainen et al., 2011; Tuppurainen and Oura, 2012). Evidence of LSDV mechanical transmission was obtained for Male Rhipicephalus appendiculatus (Tuppurainen et al., 2013a), while mechanical and transstadial transmission by Amblyommahebraeum have been demonstrated (Lubinga et al., 2015). LSDV has been detected in the saliva of both tick species by Lubinga et al. (2013). Transovarial transmission of LSDV by Rhipicephalus decoloratus was also reported (Tuppurainen et al., 2013b; Lubinga et al., 2014b). Using immunohistochemical staining, LSDV infection has been detected in various tick organs including midgut, salivary glands, ovaries, testes, and body fat (Lubinga et al., 2014a). It is necessary to understand the role of different arthropod species in LSDV transmission in order to control the spread of the disease and to provide a useful insight into the epidemiology of LSDV, consequently, the present study aims to investigate molecularly the potential role of R. annulatus in the transmission of LSDV in Egypt with a special regard to the vertical route of transmission.
Materials and methods
Animals
Three hundred native cows were included in the current study. Animals were distributed in three districts of Beni-Suef governorate, Egypt [Somosta, (150), El-Wasta, (100) and Beni-Suef, (50)].
Samples
Tick samples
All the 300 animals were examined for tick infestation.A total of 18 animals were found to harbour hard ticks. Different stages of ticks were directly picked up from animals and when possible from the nodules using pointed forceps. The ticks were identified according to (Walker et al., 2003). The collected ticks were divided into fully-engorged females, partially-engorged adults and nymphs. Fully engorged females were prepared for oviposition for obtaining eggs and then larvae, while the other adults and nymphs were preserved in ethanol 70% till used in PCR.
Skin nodules
Skin biopsies comprising epidermis, dermis and subcutis of the nodules were collected from animals infested by the ticks. The nodules were surgically extirpated after the skin was locally anesthetized with 2% lidocaine then placed in glycerol saline (sodium chloride 4.2g, dibasic potassium phosphate 3.1g, monobasic potassium phosphate 1.0g, phenol red sodium salt 0.05g, glycerin 298.5 ml, ethanol 2.5 ml, demineralized water 800.0 ml and pH 7.2 ± 0.2, 25°C) and stored at −20°C for PCR analysis.
Field Tick Oviposition and Larval Hatching
A total of six fully engorged female ticks were washed with phosphate buffer saline then placed individually (one female per tube) in glass vials and incubated at 27–28°C, 80–85% relative humidity (RH) until oviposition (Castro-Janer et al., 2009). A sample was taken from each eggs mass, then pooled and subjected to DNA extraction and virus isolation. The other eggs masses were individually incubated under the previous conditions until the larvae hatched.
Processing of Ticks for Virus Isolation and PCR
Adult ticks, nymphs and hatched larvae were collected in 1.5 ml micro tubes and rinsed three times in PBS with 0.25mg/ml gentamycin and 2x103 IU/ml polymyxin B. The washed ticks were pooled, ground in a mortar, and centrifuged at 2000 rpm for 15 minutes. The supernatant was collected and used for virus isolation and/or DNA extraction. For homogenization of eggs’ samples, the eggs were sonicated using ultrosonic processor with 2mm microtip probe. The optimum sonication was 4 cycles of 3 sec. bursts and 5 sec. interval between bursts. The homogenate was centrifuged at 600g for 5 min, and the supernatant was collected and stored at -80°C to be used for virus isolation.
Virus Isolation
The supernatant fluid from tick eggs and larvae was inoculated separately onto the chorioallantoic membrane (CAM) of 9-11 days-old fertile specific pathogen free embryonated chicken egg (SPF-ECE) according to (Michael et al., 1991). All eggs were incubated at 37°C and 70% humidity after sealing the openings with melted wax. The eggs were candled daily for five days and embryos dying during the 1st day post inoculation (PI) were discarded as non-specific deaths. On the 5th day PI, eggs with a live embryo were kept at 4 ºC overnight then the CAMs were harvested and examined for characteristic pock lesions. CAMs showing no pock lesions were subjected to one or two blind passages. The presence of LSDV was further confirmed using PCR on the infected CAM homogenates for egg and larvae.
DNA Extraction
Four pooled samples were processed from each locality. Pooling was occurred for each adult, nymphs, nodules, eggs and larvae. DNA extraction was conducted using a DNA Mini Kit (Thermo, Germany) according to the manufacturer’s instructions.
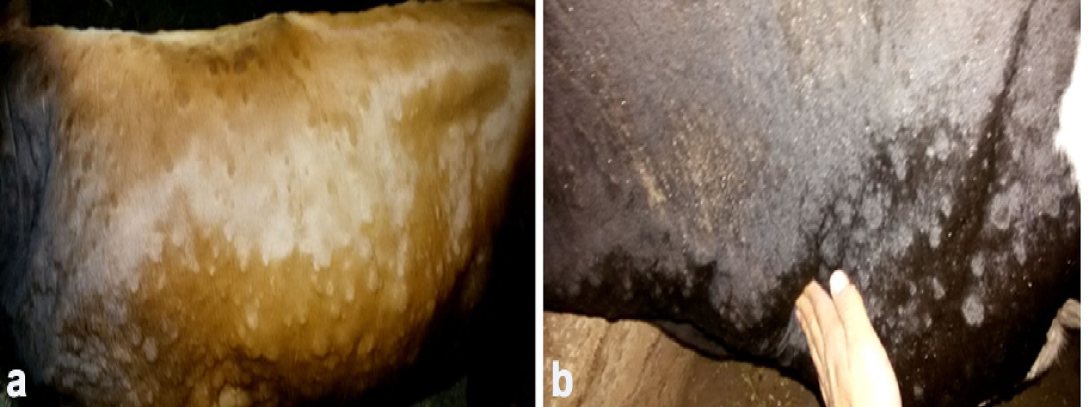
Figure 1: Clinical sings of LSD, a. Cutaneous nodules covering the entire body of a cow naturally infected with LSDV (generalized distribution). B. Enlargement of prescapular lymph node accompanied with LSD nodules in a cow infested with ticks.)
PCR and Sequencing
PCR amplification was performed using PCR master mix (Jena bioscience Gmbh, Germany) in a total volume of 25µl/reaction. The PCR primer set targets 192 bp of the P32 viral attachment gene with following sequences; forward primer: 5′-TTTCCTGATTTTTCTTACTAT-3′ and reverse primer: 5′-AAATTATATACGTAAATAAC-3′ (Ireland and Binepal, 1998). PCR amplicon was electrophored in 2% agarose gel containing ethidium bromide at 100V for 45 min and visualized in trans-illuminator. Four PCR amplicons (three from cutaneous nodules, representing the three districts in Beni-Suef, Egypt and one from adult field ticks) were extracted from the gel and purified with a gel extraction Kit (Qiagen) according to the manufacturer’s instructions. Purified gel products were sequenced commercially. BLAST analysis was initially performed to establish sequence identity to GenBank accessions. Phylogenetic tree and sequence alignments were created using MEGA 6 software (Tamura et al., 2013). A tree was constructed by the Neighbor-Joining method based on 1000 bootstrapped data sets.
Results
Clinical Signs of LSDV in Diseased Animals
Clinical examination of all infected animals revealed, pyrexia of 40.0 to 41.5°C, with lachrymation, anorexia, reported a decrease in milk production and body weight, in addition to skin nodules observed all over the body.The skin nodules were of various sizes ranging in diameter from 0.5 to 5 cm. They were raised, circumscribed, firm and accompanied by swelling of the superficial lymph nodes (Figure 1). Different stages of Rhipicephalus annulatus tick (nymph and adult) were found on the abdomen and brisket of infected animals. Eighteen Out of the three hundreds of the examined animals with clinical signs of LSD, showed tick infestation.
Virological Assay
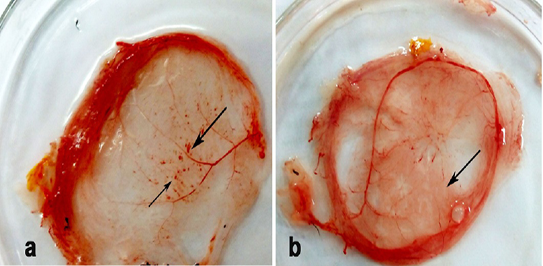
Thickening and hemorrhagic pock lesions (Figure 2) in the CAM were observed in ECE inoculated with R. annulatus eggs and larvae after two egg passages. The pock lesions became prominent after the 3rd passage.
PCR Analysis
All samples (adult ticks, nymphs and skin nodules) that were collected from the diseased cattle were tested positive for LSDV by PCR and yielded a single amplicon specific (192bp) for P32 gene. In addition, eggs, larvae and inoculated CAMs showed positive PCR results.
Sequencing of PCR Product
Sequencing revealed that the amplified 192bp PCR product from three localities (Egypt-BSU/Beni-Suef/2015, Egypt-BSU/Somosta/2015, Egypt-BSU/El-Wasta/2015 and Egypt-BSU/ R. annulatus/2015) covered the terminal 174bp of the LSDV P32 gene. Comparative P32 gene
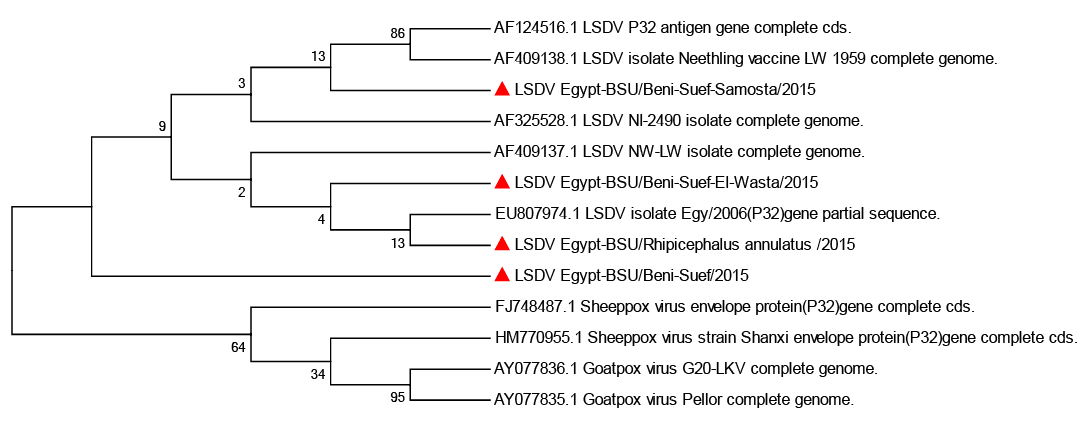
Figure 3: Phylogenetic analysis of the attachment protein (P32) gene: the tree constructed by the neighbour-joining method
sequence analysis using Capripoxvirus database available sequences showed significant nucleotide sequence homology with 97 to 100% nucleotide identity. Phylogenetic analysis revealed that the obtained LSDV sequences are in the same clade and essentially identical to Kenyan isolate NI-2490/1958 (AF325528), South African isolate NW-LW/1999 (AF409137). In contrast Egy/2006 isolate (EU807974) appeared to be in a separate clade and also, Neethling vaccine LW 1959 complete genome isolate (AF409138) appeared to be in a separate clad with LSDV P32 antigen gene complete cd (AF124516) (Figure 3).
Discussion
LSD is currently considered a vector-borne disease with a high transmissibility that can be influenced by vector population (warm & wet season), vector mobility, blood-feeding (transcutaneous) and biological cycle in ticks (Tuppurainen et al., 2011; Tuppurainen et al., 2013b; Lubinga et al., 2014c). Improved understanding of the distribution and population dynamics of the vectors is the corner stone for the assessment and the management of the risks associated with LSD. It has previously been reported that after feeding on experimentally infected cattle, R. decoloratus females and subsequently their eggs tested positive for LSDV (Tuppurainen et al., 2011). Moreover, LSDV nucleic acid has been detected in naturally infected ticks, collected from clinically affected animals from Egypt and South Africa with high infection rates (33–100%) (Tuppurainen et al., 2015).
The current study was conducted to determine the ability of R. annulatus ticks collected from LSDV naturally infected animals to transmit LSDV transovarially based on PCR and virus isolation.
Clinical examination of animals located in the area under investigation revealed that the affected animals suffered from fever, nasal secretions, salivation and skin nodules distributed all over the entire body as well as enlarged superficial lymph nodes. All these signs are characteristic for LSD and in agreement with those previously illustrated by (Davies, 1991; Coetzer, 2004). It is worth mention that animals were suffering from decreased weight gain not only due to LSDV infection as described by (Woods, 1988) but also due to the burden of Rhipicephalus ticks. The latter are considered the most damaging cattle parasite in Africa and have a negative impact on the cattle health and productivity. It has been estimated that an infestation with 50 or more engorged Boophilus female ticks causes an annual weight reduction of 0.5 kg per tick. Moreover, reduction of the annual milk production can go up to 200 liters per animal in dairy cows1.
Although the tentative diagnosis of LSD is usually based on the presence of the characteristic clinical signs, a rapid sensitive laboratory method such as PCR is required to confirm the infection (Ireland and Binepal, 1998; Heine et al., 1999; Balinsky et al., 2008; Awad et al., 2009). Skin nodules collected from animals showed positive PCR results for LSDV. Adult and nymph ticks collected from diseased animal were also tested positive for LSDV using PCR test. Multi sequence alignment and phylogenetic analyses revealed significant sequence homology of LSDV sequences obtained to Gen-Bank available Capripoxvirus sequences with 97 to 100% nucleotide identity. Preservation of adult tick and nymphs in ethanol 70% prior to testing by PCR makes the possibility of the ticks being contaminated with virus from the surface of the nodules on the infected animals is highly unlikely. Also washing the fully engorged female ticks three times prior to oviposition helped to reduce possible surface contamination in order to decreasing the probability of false positive reaction.
Engorged-female ticks collected from areas above skin lesions were selected for oviposition. LSDV nucleic acid was detected in eggs laid by the female ticks suggesting that the virus in the blood meal survived and was able to reach the ovaries. This was previously discussed for other viruses by (Booth et al., 1989, 1991; Sonenshine, 1991; Labuda and Nuttall, 2004) and confirmed by (Lubinga et al., 2014a) who demonstrated LSDV using immunohistochemical staining in various tick organs including mid gut, salivary glands, ovaries, testes, and body fat. Moreover, evidence of trans-ovarial passage of LSDV in R. decoloratus has been reported (Tuppurainen et al., 2011) which strengthens our results.
Detection of LSDV in larvae proves the possibility of tick’s larva for being a potential source of infection for susceptible animals. It has previously been reported that infected R. decoloratus females were able to pass the infection vertically, via their eggs, larvae. The latter were able to transmit the virus to their bovine hosts thus causing mild clinical LSD in naïve recipient animals (Tuppurainen et al., 2013b).
Rhipicephalus annulatus is a hard tick found most often on cattle in subtropical and tropical regions and is widely distributed in Egypt (Walker et al., 2003). Since R. annulatus is a one-host tick, of which all stages feed on the same animal (Walker et al., 2003), transmission of pathogens between hosts is likely to be vertical through a transovarial passage. In summer, R. annulatus ticks can survive for as long as 3-4 months without feeding. In cooler temperatures, they may live without food for up to six months which gives the tick the potential to be a reservoir for the pathogen. Furthermore, the period of infestation of cattle is approximately three weeks. The life cycle can be completed in two months, and six generations per year are possible under conditions of continuous high temperature and humidity; this characteristic can lead to a heavy tick burden on animals (Walker et al., 2003).
It is worthy mention that only 18 out of 300 clinically infected animals were found to harbour ticks, the number of infested cattle with ticks is highly influenced by the attempts of ticks control done by the owner which directly affects the number of the collected samples and their viability.
Conclusion
In the present study, it was shown that infected R. annulatus females are able to pass the infection vertically, via their eggs to the larvae. The findings of this study indicates a high possibility for ticks to be a reservoir host for LSDV in nature and a considerable risk that requires a further investigation and more in depth analysis.
Conflict of interests
The authors declare no conflict of interest.
Acknowledgements
This study was supported by a grant awarded by the Unit of Funding Researches and Projects, Beni-Suef University, Egypt.
References