A Review of 20 Years Research Progression on Prodrug Technology in Inhaled Medications
A Review of 20 Years Research Progression on Prodrug Technology in Inhaled Medications
Lijuan Liu1, Jinghang Chen2*, Min Wang3 and Wei Li4
Prodrugs to improve physicochemical properties.
Prodrugs to reduce local and systemic toxicity. G4 PAMAM, Generation 4 polyamidoamine.
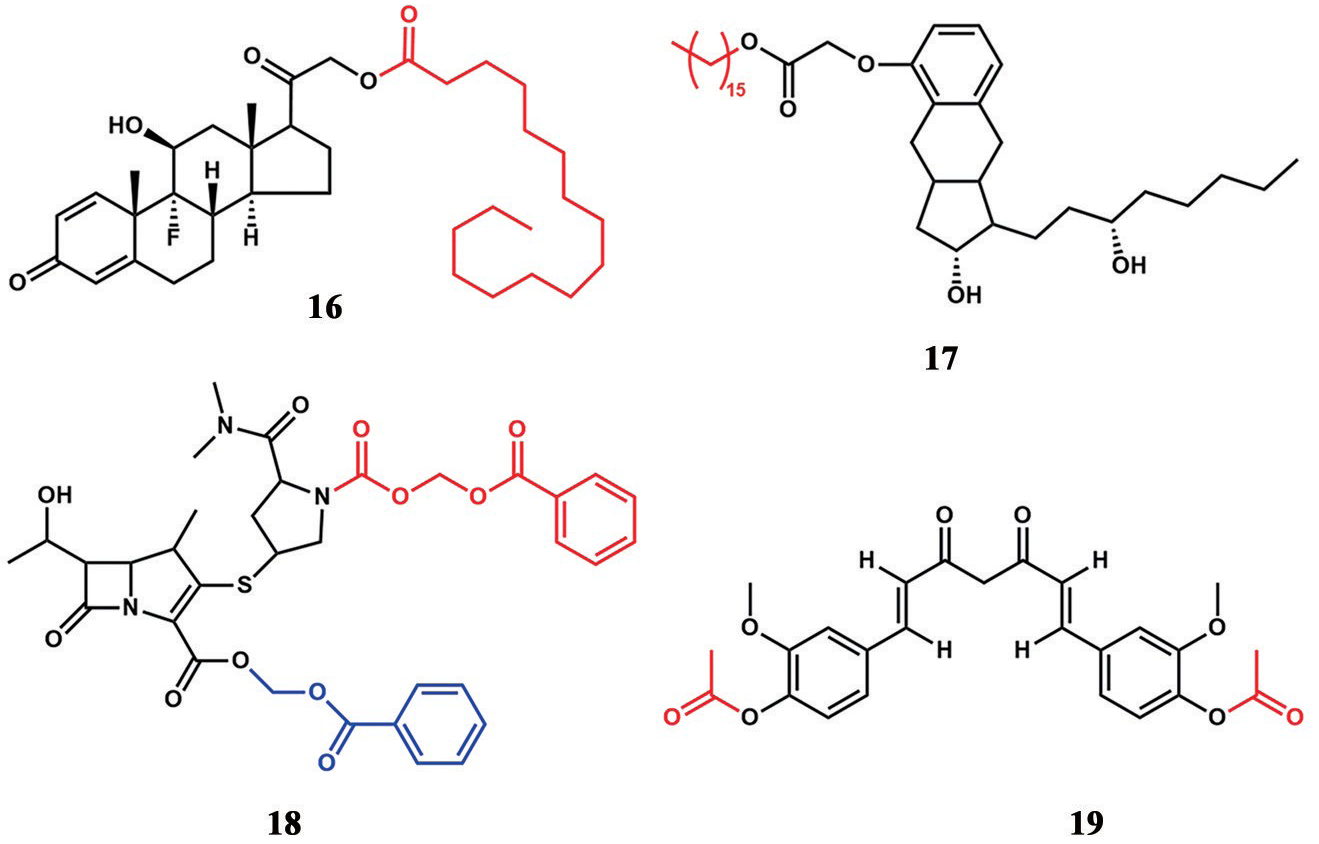
Esterified prodrugs to prolong lung retention.
Macromolecular prodrugs to prolong lung retention. PTX, Paclitaxel; IFN, Interferon; PEG, Polyethylene glycol; HSA, Human serum albumin; Sulfo-SMCC, Sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclo-hexane-1-carboxylate.
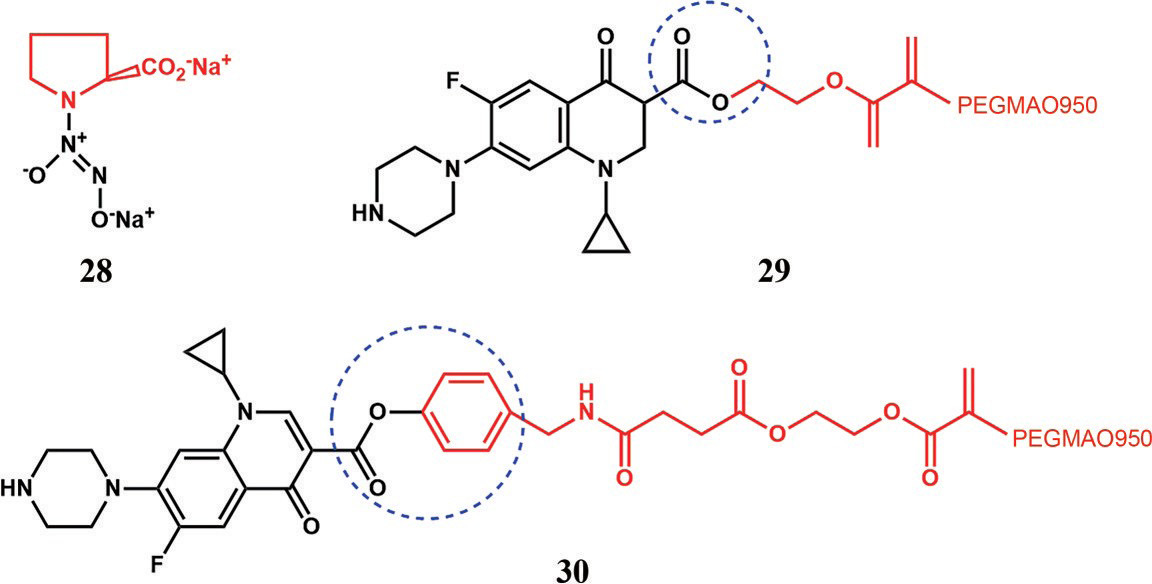
Other prodrugs to prolong lung retention. PEGMAO950, Polyethylene glycol methacrylate.
Prodrugs for actively targeted delivery.
Biologically activated prodrugs for targeted drug release. DOX, Doxorubicin; PEI, Polyethylenimine.
Prodrugs for pulmonary delivery of peptides and pro- teins. Cys, Cysteine; Lys, Lysine; Pro, Proline; His, Histidine.
Inhaled bioprecursors.
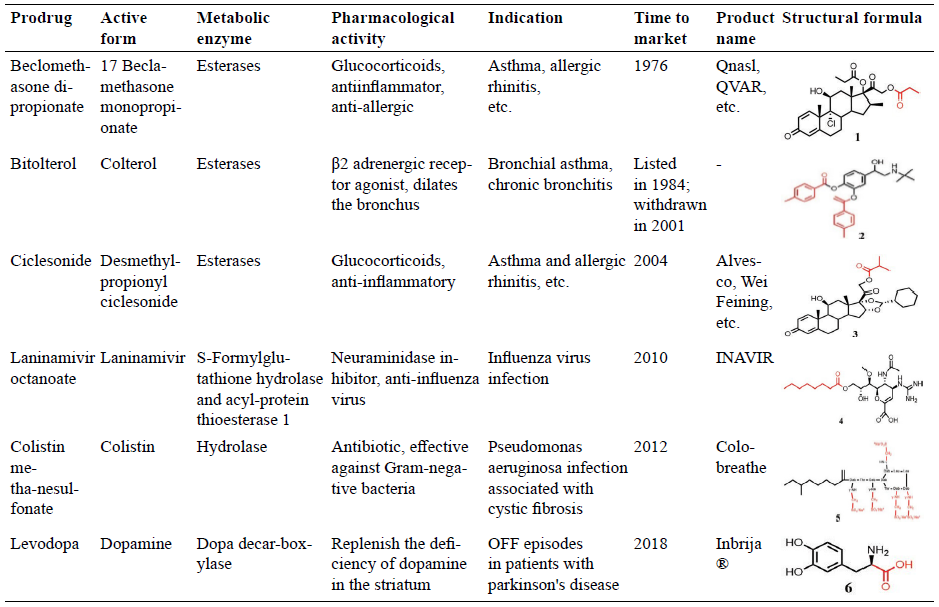
Marketed inhalation prodrugs.