Administration of Encapsulated of Lactobacillus Plantarum and Peperomia Pellucida Plant Extract on Performance, Protein Digestibility and Immunity of Broiler Chickens
Research Article
Administration of Encapsulated of Lactobacillus Plantarum and Peperomia Pellucida Plant Extract on Performance, Protein Digestibility and Immunity of Broiler Chickens
Muhammad Luthfi Hidayat, Istna Mangisah, Lilik Krismiyanto, Vitus Dwi Yunianto*
Department of Animal Science, Faculty of Animal and Agricultural Sciences, Universitas Diponegoro Jalan Prof. Soedarto, SH, Tembalang, Semarang, Central Java, Indonesia.
Abstract | This study aims to determine the effects of administration of encapsulated Lactobacillus plantarum and Peperomia pellucida plant extract (ELPPP) on performance, protein digestibility and immunity of broiler chickens. A total of 200 Ross strain broilers were arranged in a completely randomized design with 5 treatment groups and 4 replications. The treatments include: T0 (control), T1 (0.1% ELPPP), T2 (0.2% ELPPP), T3 (0.3% ELPPP), T4 (0.4% ELPPP). ELPPP was applied from day 8 to 35. The parameters observed included broiler performance, bacterial populations in the small intestine, small intestine pH, protein digestibility, malondialdehyde (MDA), superoxide dismutase (SOD), H/L ratio and relative weight of immune organs. The results showed that ELPPP increased (p <0.05) performance, small intestine bacterial balance, protein digestibility, SOD level, relative weights of immune organs, but decreased small intestinal pH, MDA levels and H/L ratio. However, there was no significant effect (p >0.05) on ration feed consumption. In conclusion, the administration of ESCLP improved the performance, small intestine bacterial balance, protein digestibility, and immune system of broiler chickens.
Keywords | Antioxidant, Broiler growth, Intestial microbial, Probiotic, Phytobiotic
Received | June 09, 2024; Accepted | July 18, 2024; Published | August 23, 2024
*Correspondence | Vitus Dwi Yunianto, Department of Animal Science, Faculty of Animal and Agricultural Sciences, Universitas Diponegoro Jalan Prof. Soedarto, SH, Tembalang, Semarang, Central Java, Indonesia; Email: v.dbi@gmail.com
Citation | Hidayat ML, Mangisah I, Krismiyanto L, Yunianto VD (2024). Administration of encapsulated of lactobacillus plantarum and peperomia pellucida plant extract on performance, protein digestibility and immunity of broiler chickens. Adv. Anim. Vet. Sci. 12(10): 1896-1902.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.10.1896.1902
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Broiler chicken is a strain of chicken that has superior genetic and productivity, especially in meat production. However, rearing in a tropical environment has its own challenges, because broilers are sensitive to high ambient temperatures, so the potential for exposure to stress is very high (Kpomasse et al., 2021). Stress can have a negative impact on the physiology, metabolism, immunity and performance of broiler chickens (Kikusato and Toyomizu, 2023). Nutritional manipulation such as feed additive supplementation is one strategy to overcome the adverse effects of stress (Abdel-Moneim et al., 2021). Since the ban on antibiotic growth promoter (AGP), the use of natural feed additives such as a combination of probiotics and phytobiotics can be an alternative to AGP (Sapusha et al., 2021).
The use of probiotics from Lactobacillus plantarum is known to provide beneficial effects for broiler chickens. Several studies have shown positive effects of Lactobacillus plantarum feeding on immune status, gut microecological balance and performance in broiler chickens (Wang et al., 2023). Additionally, several studies have shown that the combined administration of probiotics and phytobiotics has a better impact on chickens compared to separate administration (Yuanita et al., 2019; Sapsuha et al., 2021). Ferdous et al. (2019) also reported that probiotics combined with phytobiotics were able to reduce intestinal pH which has an impact on reducing coliforms and increasing the performance of broiler chickens. Peperomia pellucida plant extract can be used as a phytobiotic. Peperomia pellucida plant extract contains phenolics (627.45 mg/g), flavonoids (868.78 mg/g) and tannins (13.06 mg/g) (Fakayode et al., 2021). Phenolic compounds act as a source of antioxidants and antimicrobials (Srivastava et al., 2023). Several studies have shown that phenolic compounds and flavonoids contained in phytobiotics can increase the activity of antioxidant enzymes in broiler chickens (Kikusato, 2021) so as to minimize the negative effects of stress. In addition, polyphenols contained in phytobiotics can support the growth of probiotic bacteria (Prakasita et al., 2019). Therefore, the combination of Lactobacillus plantarum and peperomia pellucida plant extract is expected to synergize with each other.
Table 1: Composition and nutrient contents of basal feed.
|
Ingredients |
Compositions |
|
|
Starter (8-21) |
Finisher (22-35) |
|
|
Corn |
50.41 |
55.41 |
|
Rice bran |
14.74 |
14.74 |
|
Soybean meal |
24.00 |
19.00 |
|
Meat bone meal |
10.00 |
10.00 |
|
CaCO3 |
0.30 |
0.30 |
|
Premixes |
0.25 |
0.25 |
|
Lysine |
0.1 |
0.1 |
|
Methinonine |
0.20 |
0.20 |
|
Total |
100.00 |
100.00 |
|
Nutrient content |
||
|
Metabolizable energy (kcal/kg) |
3,062 |
3.073 |
|
Crude protein (%) |
21.63 |
19.68 |
|
Crude fiber (%) |
4.37 |
4.45 |
|
Ether extract (%) |
3.39 |
4.33 |
|
Calcium (%) |
1.44 |
1.42 |
|
Phosphorus (%) |
0.75 |
0.72 |
1Result of Analysis of Laboratory of Nutrition and Feed Science, Faculty of Animal and Agricultural Sciences, Diponegoro University, Semarang;2Metabolizable energy was calculated based on formula (Bolton 1967) as follows: 40.81 [0.87 (Crude Protein + 2.25 crude fat + nitrogen-free extract) + 2,5].
On the other hand, secondary metabolite compounds contained in plant extracts (Sugihato and Ayasan, 2022) and probiotic viability (Dumitru et al., 2021) are highly susceptible to storage, light, pH, temperature, heat and oxygen. This causes the stability and availability of these bioactive compounds to be low, thus reducing their effectiveness against their host (Agriopoulou et al., 2023). Encapsulation is effective in protecting the active compounds contained in probiotics and plant extracts so that the release of active compounds can be controlled according to the targeted organ (Klojdova et al., 2023). In addition, encapsulation also helps to mask the unpleasant taste and smell of the compound so that it does not affect the palatability of the chicken (Agustyaningsih et al., 2022). To our knowledge, there is no literature regarding the encapsulated mixture of Lactobacillus plantarum and Peperomia pellucida plant extract. Therefore, this research aims to determine the effect of adminitrastration encapsulated Lactobacillus plantarum and Peperomia pellucida plant extract on the performance, protein digestibility and immunity of broiler.
MATERIALS AND METHODS
Experimental Animals and Diets
This research was supervised and was approved by the Animal Research Ethics Committee, Faculty of Animal and Agricultural Sciences, Diponegoro University under approval number 60-07/A-14/KEP-FPP. A total of 200 Ross broilers chicks with an average body weight of 45,16 ± 0,10 g from a commercial hatchery were used in this study. The chicks were raised in a broiler house with a litter floor (rice husk) and manual feeders and drinkers. The chickens were raised for 35 days and given commercial diet and basal diet. The basal diet is formulated according to the recommended requirement standards by NRC (1994) in Table 1. Commercial diet (pre-starter) was given at the age of 1-7 days and basal diet (starter and finisher) and ELPPP was given at the age of 8-35 days. ELPPP are given as percentages, based on feed consumption. Treatment dosage selection based on research Yuanita et al. (2019).
Experimental Design and Treatments
A completely randomized design was used in this study. On day 8, broiler chicks (weight 175.60 ± 3.16 g) were randomly allocated into 5 treatments and 4 replicates (each experimental unit contained 10 chicks). The treatments were as follows:
T0: basal feed without encapsulated Lactobacillus plantarum and Peperomia pellucida plant extract (ELPPP)
T1: basal diet + 0.1% ELPPP
T2: basal diet + 0.2% ELPPP
T3: basal diet + 0.3% ELPPP
T4 : basal diet + 0.4% ELPPP
Preparation of Encapsulated of Lactobacillus Plantarum and Peperomia Pellucida Plant Ectract
Peperomia pellucida plants were obtained around Diponegoro University. The collected Peperomia pellucida was weighed, washed and dried. The drying process uses an oven with a temperature of 50 ° C and is ground until powder is obtained. Peperomia pellucida plant extract was made based on the method described by (Gouda et al., 2021). The extract was dissolved in distilled water in a ratio of 3:1 (ml:ml). Preparation of Lactobacillus plantarum solution (1010 cfu/ml) starts with dissolving skim milk as much as 10% of the distilled water solution. After that, the skim milk solution and Lactobacillus plantarum were mixed with Lactobacillus plant arum (2:1, ml;ml). Peperomia pellucida extract solution was mixed with Lactobacillus plantarum (1:1, ml;ml). Then encapsulation was carried out.
The encapsulation of the mixture of Lactobacillus plantarum and Peperomia pellucida plant extract refers to the method of Agustyaningsih et al. (2022). The coating material used for encapsulation was maltodextrin. Maltodextrin was dissolved in distilled water in a ratio of 1:3 (ml:ml). The dissolved maltodextrin was mixed with a mixture of Lactobacillus plantarum and peperomia pellucida extract in a ratio of 1:5 (ml:ml) until homogeneous and freeze drying was carried out to obtain an encapsulated mixture of Lactobacillus plantarum and Peperomia pellucida plant extract. The encapsulated mixture of Lactobacillus plantarum and Peperomia pellucida plant extract was stored in the refrigerator until use.
Animals Rearing Management
During rearing, all experimental units were in a healthy condition and received the same treatment of both temperature and humidity. Temperature, humidity and lighting program were set up as it recommended in Ross management manual. Chickens were firstly weighed to determine initial body weight, and subsequent weighing was performed once a week. Feed and drinking water were provided ad libitum during the rearing period. The Chickens were vaccinated newcastle disease (ND) and infectious bronchitis (IB) at after hatch with 0,1 ml spray technique and Gumboro/Infectious Bursal Disease (IBD) vaccine (Medivac Gumboro B vaccine) is given at 12 days of age as one dose through drinking water.
Sampling And Parameter Measurement
Performance of Broiler: Body weight was obtained by weighing the chickens at 35 days of age. Body weight measurements are carried out once a week. Body weight gain was obtained by subtracting the final weight from the initial weight of the treatment. Feed intake was recorded daily by subtracting the total feed given from the remaining feed. Feed conversion ratio was calculated by dividing total feed intake by body weight gain.
Microbial Population: Bacterial populations in the small intestine (lactic acid bacteria and coliforms) were measured based on the method of (Sugiharto et al., 2017). Samples of small intestine digesta from each replicate were taken and put into a sterile cup. Then the digesta samples were analyzed for total bacteria (LAB and Coliform) using the total plate count method. MacConkey agar medium (Merck KGaA) was used to grow coliform bacteria and incubated anaerobically for 24 hours at 38°C. The colonies of coliform bacteria counted were red and colorless. The number of lactic acid bacteria was counted on MRS agar (Merck KGaA) after aerobic incubation at 38°C for 48 hours.
pH of Small Intestine: The pH of the small intestine of each experimental unit was measured by taking a sample of chicken small intestine digesta at 35 days of age and measured with a digital pH meter.
Protein Digestibility: Protein digestibility test was conducted for 4 days at the age of 32-35 days and measured by a combination of total collection method and indicator (Fe2O3) based on the method of (Afro et al., 2023). During data collection, chickens were placed in battery cages with 20 excreta collection units filled with one chicken. The Fe2O3 indicator is 0.5% of the ration, given alternately on the first and third days. On the first day, chickens were given the treatment ration that had been added with the indicator for 24 hours. Excreta collection began when the feces first came out red until the second day changed color. This was done until the fourth day. Every day, feces were collected and sprayed with 0.2 N HCL every 2 hours (Krismiyanto et al., 2022). The collected excreta were weighed, dried and analyzed for crude protein content using the Kjeldahl method. Calculation of protein digestibility based on the formula according to McDonald et al. (1997).
Superoksida dismutase (SOD) dan Malondialdehyde (MDA): Blood serum from broiler chickens aged 35 days in each replication was taken for superoxide dismutase (SOD) and malondialdehyde (MDA) tests based on the method of (Agusetyaningsih et al., 2022). SOD activity was tested according to the ability of the sample to suppress the auto-oxidation of pyrogalol. The mixture used consisted of samples, 50 mM Tris-HCl (pH 8.2) and 1 mM pentaacetic acid diethylenetriamine. Pyrogalol (final concentration 0.2 mM) was added to determine the reaction and the absorption value was calculated kinetically. The concentration of SOD was stated in units of U/ml. MDA activity was measured using the thiobarbituric acid (TBA) reactive substance test. Samples were vortexed, 8.1% sodium dodecyl was added, and left at room temperature for 10 minutes. Control samples were done in the same way. After incubation, the samples were added 20% acetic acid and 0.6% TBA. Then the tubes were placed in a water bath at 90-95°C for 1 hour. After that, butanol:pyridine (15:1) was applied to the supernatant. Then the mixture will be vortexed and centrifuged. MDA concentration is expressed as nmol/ml.
Heterofil-limfosit Ratio: Measurement of the heterophil/lymphocyte ratio (H/L) was carried out using the leukocyte differential observation method based on the method described by Sunu et al. (2021). Measurement of leukocyte differential was done by making a blood smear preparate. Blood samples were taken on day 35 of each replicate and put in EDTA tubes. Blood smears were made on object glass, then fixed with methanol, stained with giemsa, washed with water and allowed to dry at room temperature. The finished smear preparation was observed with a microscope and the percentage of heterophils and lymphocytes obtained was calculated. Then the percentage of heterophils and lymphocytes was multiplied by the number of leukocytes to obtain the number of heterophils and lymphocytes. Determination of the H/L ratio was obtained by dividing the number of heterophils by the number of lymphocytes.
Table 2: Performance of 35-day-old broilers.
|
Variabel |
Treatment |
SEM |
p- value |
||||
|
T0 |
T1 |
T2 |
T3 |
T4 |
|||
|
Body weight (g) |
1651d |
1717c |
1884b |
1908ab |
1952a |
27.57 |
0.000 |
|
Weight gain (g) |
1477d |
1543c |
1711b |
1732ab |
1773a |
27.28 |
0.000 |
|
Feed intake (g) |
2567 |
2445 |
2644 |
2595 |
2608 |
26.03 |
0.131 |
|
Feed convertion |
1.73a |
1.58b |
1.54b |
1.49b |
1.47b |
0.026 |
0.01 |
a. b. c. d means in the same row with superscripts are significantly different (p <0,05).
Relative Weight of Immune Organs: Measurement of the relative weight of immune organs of each replicate was carried out on day 35. Chickens were slaughtered and removed immune organs (Bursa fabricius, thymus and spleen), then weighed and expressed as a percentage of live weight according to Fajrih et al. (2014).
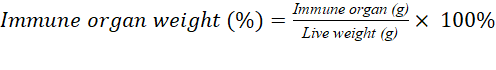
Statistical Analysis
The data has met the assumptions of normality and homogeneity tests. The data were analyzed by analysis of variance (ANOVA) using SPSS software and if there was a significant effect then continued with the Duncan test.
RESULTS AND DISCUSSION
Performance of Broilers
The results of statistical analysis showed that the provision of encapsulated Lactobacillus plantarum and Peperomia pellucida plant extract (ELPPP) in this study (Table 2) had a positive effect on increasing (p<0.05) body weight gain by 1.03 (T1), 1.14 (T2), 1.15 (T3) and 1.18 (T4) times that of the control and broiler body weight gain by 1. 09 (T1), 1.12 (T2), 1.16 (T3) and 1.20 (T4) times that of the control. It also reduced (p <0.05) the feed conversion ratio by 1.09 (T1), 1.12 (T2), 1.16 (T3) and 1.17 (T4) times that of the control. However, feed intake was not affected (p >0.05) the treatment.
This research shows that administration ELPPP can increase body weight and body weight gain. Several studies have shown that giving a mixture of probiotics and phytobiotics to broiler chickens can increase broiler body weight (Ferdous, 2019; Sapsuha et al., 2021). In this case, it is possible that the metabolite compounds contained in the mixture of Lactobacillus plantarum and Peperomia pellucida plant extract that are encapsulated are optimally absorbed so that it can be utilized properly. Natsir et al. (2019) reported that encapsulation treatment was more effective in improving intestinal performance, ecology and morphology compared to non-encapsulation treatment. There was no significant (p >0.05) difference in feed intake in this study. This may occur because encapsulation can mask the taste and smell of ELPPP so that it does not affect palatability and feed intake. Sugiharto and Ayasan. (2022) reported that undesirable taste and smell can be masked by encapsulation. However, this providing evidence that the synergistic effect of ELPPP can improve feed efficiency, as indicated by a lower feed conversion ratio compared to the control. This means that the nutrients in the feed are optimally absorbed. Sapsuha et al. (2021) reported that probiotics and phytobiotics have pharmacological effects as antibacterials that can improve intestinal health so that the absorption of nutrients is optimal and has an impact on improving broiler performance.
Table 3: Microbial population, pH of small intestine and protein digestibility.
|
Variable |
Treatment |
SEM |
p-value |
||||
|
T0 |
T1 |
T2 |
T3 |
T4 |
|||
|
LAB (log cfu/g) |
8.73c |
9.18b |
9.30ab |
9.40ab |
9.49a |
0.37 |
0.000 |
|
Coliforms (log cfu/g) |
2.81a |
2.57b |
2.51bc |
2.45bc |
2.39c |
0.68 |
0.000 |
|
pH |
6.51a |
6.35b |
6.22c |
6.21c |
6.10c |
0.36 |
0.000 |
|
Protein digestibility |
78.94c |
82.92b |
83.10ab |
84.10ab |
86.00a |
0.62 |
0.000 |
a. b. c means in the same row with superscripts are significantly different (p < 0.05).
Microbial Population AND Protein Digestibility
Data on microbial population, small intestine pH, and protein digestibility in treated broiler chickens are presented in Table 3. ELPPP feeding increased (p <0.05) LAB population by 1.05 (T1), 1.07 (T2), 1.08 (T3) and 1.09 (T4) times that of the control and protein digestibility by 1.05 (T1), 1.05 (T2), 1.07 (T3) and 1.09 (T4) times that of the control. It also reduced (p <0.05) coliform population by 1.09 (T1), 1.12 (T2), 1.15 (T3) and 1.18 (T4) times that of the control and small intestinal pH by 1.03 (T1), 1.05 (T2), 1.05 (T3) and 1.07 (T4) times that of the control.
The results of this study showed that chickens given ELPPP had a high (p <0.05) population of lactic acid bacteria (LAB) and there was a decrease (p <0.05) in the population of coliform compared to controls. This condition may be due to the antibacterial activity contained in ELPPP. Thus, beneficial bacteria are able to compete for nutrients in the small intestine through competitive exclusion mechanisms with pathogenic bacteria. Through this mechanism, beneficial bacteria can help maintain the balance of the intestinal microbiota. In addition, the decrease (p <0.05) in pH in this study played a role in improving the balance of bacteria in the small intestine. This study is in line with Sapsuha et al., (2023) who showed a decrease in pH in the small intestine when given a mixture of nutmeg extract and Lactobacillus plantarum. The decrease in pH in the small intestine seems to be caused by organic acids or short chain fatty acids (SCFA) produced by Lactobacillus plantarum, although SCFA levels were not measured. Vimon et al. (2023) that lactic acid and SCFA caused a condition of the intestine to become acidic, which affected the decrease in the intestinal pH. In addition, acidic pH conditions in the digestive tract are needed as a precursor to increase the activity of digestive enzymes (Pearlin et al., 2020). This was proven to increase (p <0.05) protein digestibility in this study. The increased LAB condition allows protein to be absorbed optimally. Mangisah et al. (2020) reported that increasing LAB has a positive impact on intestinal health and the growth of intestinal villi, which indirectly improves nutrient digestibility and absorption. LAB also produce proteolytic enzymes that are useful in digesting protein (Kieliszek et al., 2021).
Immune Response
The Administration of ELPPP (Table 4) to the diet increased (p <0.05) the relative weight of bursa by 1 (T1), 1.15 (T2), 1.17 (T3) and 1.20 (T4) times that of the control and thymus by 1.41 (T1), 1.64 (T2), 1.72 (T3) and 1.76 (T4) times that of the control. But had no effect (p >0.05) on the relative weight of spleen (Table 4).
The results of this study showed a significant (p <0.05) increase in the relative weight of the bursa and thymus compared to the control. These results are consistent with the study by Mangisah et al. (2021) that the administration of phytobiotics and probiotics increased the relative weight of the bursa fabricius. It has a positive effect on improved immunity. In some cases, the ability and function of the immune system in chickens can be seen from the relative weight of immune organs (Sugiharto et al., 2017). The metabolite compounds contained in ELPPP may contribute to increased number of immune cells and improved immune response thus increasing the relative weight of the fabricius and thymus bursa. In addition, increased balance of intestinal microflora is able to stimulate improved immune response (Liu et al., 2022) (Table 4) thereby reducing the work of immune organs. Interestingly, the relative weight of the spleen in this study was not significantly different (p >0.05). It seems that the bursa and thymus have been able to overcome the negative effects of oxidative stress so that antigens entering the blood are minimal and the work of the spleen becomes lighter. Fajrih et al. (2014) reported that the high amount of antigen entering the spleen causes the spleen to work harder, resulting in an increase in the size of the spleen.
Table 4: Relative weight of immune organs.
|
Variable |
Treatment |
SEM |
p-value |
||||
|
T0 |
T1 |
T2 |
T3 |
T4 |
|||
|
Bursa of fabricius |
0.078b |
0.078b |
0.090bc |
0.092a |
0.094a |
0.002 |
0.019 |
|
Spleen |
0.118 |
0.129 |
0.131 |
0.132 |
0.133 |
0.003 |
0.680 |
|
Thymus |
0.265b |
0.375a |
0.436a |
0.457a |
0.467a |
0.207 |
0.001 |
a. b. c means in the same row with superscripts are significantly different (p <0,05).
There was a significant difference (p <0.05) between broilers fed ELPPP compared to the control. Data on superoxide dismutase (SOD), malondialdehyde (MDA) and H/L ratio of broiler chickens are presented in Table 5. SOD levels increased (p <0.05) by 2.72 (T1), 3.09 (T2), 3.18 (T3) and 3.15 (T4) times that of the control. It also a decrease (p <0.05) in MDA levels by 1.41 (T1), 1.58 (T2), 1.70 (T3) and 1.83 (T4) times that of the control and a decrease (p <0.05) in H/L ratio by 1.46 (T1), 1.52 (T2), 1.54 (T3) and 1.85 (T4) times that of the control.
|
Variable |
Treatment |
SEM |
p- value |
||||
|
T0 |
T1 |
T2 |
T3 |
T4 |
|||
|
SOD (U/ml) |
5.61c |
15.30b |
17.34bc |
17.85bc |
19.89a |
1.22 |
0.000 |
|
MDA (nmol/ml) |
4.25a |
3.01 b |
2.68bc |
2.49bc |
2.31c |
1.79 |
0.000 |
|
H/L Ratio |
1.11a |
0.76b |
0.73bc |
0.72bc |
0.60c |
0.428 |
0.000 |
a. b. c means in the same row with superscripts are significantly (p <0,05).
This study shows that the administration of ELPPP in feed is able to increase SOD levels and decrease MDA levels in broiler chickens. These findings are consistent with those reported by Dong et al. (2019) that there was a decrease in MDA levels and an increase in antioxidant enzymes in chickens treated with probiotics and phytobiotics. The antioxidant content, especially flavonoids contained in ELPPP, may provide protection against the negative effects of oxidative stress during maintenance. SOD plays a role in providing protection from free radicals. This causes the MDA levels of broiler chickens to be lower in the administration of ELPPP.
The increased in SOD levels in this study, seems to have a positive effect on immune enhancement, which is marked a low the H/L ratio (p <0.05). Low H/L ratio values in ELPPP feeding indicate that chickens experience mild stress. Changes in the H/L ratio were associated with the level of stress experienced by the chickens (Sugiharto et al., 2017). in this case, the ELPPP-treated feed helped to reduce oxidative stress. Several studies have shown that the administration of phytobiotics to chickens experiencing oxidative stress can reduce H/L ratio levels (Isroli et al., 2017). Changes in SOD, MDA (Saracila et al., 2021) and H/L ratio (Mohammed et al 2021) are a response to oxidative stress caused by excessive production of reactive oxygen species (ROS) in the body.
CONCLUSIONS AND RECOMMENDATIONS
The administration of encapsulated lactobacillus plantarum and Peperomia pellucida plant extract extracts (ELPPP) improves broiler chicken performance, small intestine bacterial balance, protein digestibility, and immune system of broiler chickens. The optimal dosage for T4 treatment (basal diet + 0.4% ELPPP).
ACKNOWLEDGEMENTS
Research Program of Faculty of Animal and Agricultural Sciences, Universitas Diponegoro
NOVELTY STATEMENT
The novelty of this research is the use of encapsulated Lactobacillus plantarum and Peperomia pellucida plant extract in broiler chicken
AUTHOR’S CONTRIBUTIONS
Muhammad Luthfi Hidayat: Compiled the research concept, methodology, collected and processed research data and compiled the article.
Istna Mangisah: Supervised this research.
Lilik Krismiyanto: Conducted data curation, methodology and supervised this research.
Vitus Dwi Yunianto: Conceptualized and supervised this research.
Conflict of Interest
The authors declare that there is no conflict of interests in the publication of this article.
REFERENCES
Abdel-Moneim AME, Shehata AM, Khidr RE, Paswan VK, Ibrahim NS, El-Ghoul AA, Aldhumri SA, Gabr SA, Mesalam NM, Elbaz AM, Elsayed Ma, Wakwak MM, Ebeid TA (2021). Nutritional manipulation to combat heat stress in poultry–A comprehensive review. J. Therm. Biol., 98: 1-19. https://doi.org/10.1016/j.jtherbio.2021.102915
Agriopoulou S, Tarapoulouzi M, Varzakas T, Jafari, SM (2023). Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms, 11(12): 1-25. https://doi.org/10.3390/microorganisms11122896
Afro R, Ismadi VDYB, Krismiyanto L, Mulyono M (2023). Addition of soybean meal extract with Lactobacillus plantarum in rations on protein digestibility and performance of broiler chickens. J. Indones. Trop. Anim. Agric., 48(4): 322-336. https://doi.org/10.14710/jitaa.48.4.322-336
Agusetyaningsih I, Widiastuti E, Wahyuni HI, Yudiarti T, Murwani R, Sartono TA, Sugiharto S (2022). Effect of encapsulated Cosmos caudatus leaf extract on the physiological conditions, immune competency, and antioxidative status of broilers at high stocking density. Ann. Anim. Sci., 22(2): 653-662. https://doi.org/10.2478/aoas-2021-0043
Bolton W (1967). Poultry Nutrition. MAFF Bulletin No.174. HMSO, London.
Dumitru M, Vodnar DC, Elemer S, Ciurescu G, Habeanu M, Sorescu I, Georgecu E, Dudu A (2021). Evaluation of non-encapsulated and microencapsulated lactic acid bacteria. Appl. Sci., 11(21): 1-15. https://doi.org/10.3390/app11219867
Dong ZL, Wang YW, Song D, Wang WW, Liu KB, Wang L, Li AK (2019). Effects of microencapsulated probiotics and plant extract on antioxidant ability, immune status and caecal microflora in Escherichia coli K88-challenged broiler chickens. Food Agric. Immunol., 30(1): 1123-1134. https://doi.org/10.1080/09540105.2019.1664419
Fajrih N, Suthama N, Yunianto, VD (2014). Body resistance and productive performances of crossbred local chicken fed inulin of dahlia tubers. Media Peternakan, 37(2): 108-108. https://doi.org/10.5398/medpet.2014.37.2.108
Fakayode AE, Imaghodor FI, Fajobi AO, Emma-Okon BO, Oyedapo OO (2021). Phytonutrients, antioxidants and anti-inflammatory analysis of peperomia pellucida. J. Med. Pharm. Allied Sci., 10(5): 3517-3523. https://doi.org/10.22270/jmpas.V10I5.1511
Ferdous MF, Arefin MS, Rahman MM, Ripon MM , Rashid MH, Sultana MR, Hossain MT, Ahammad M, and Rafiq K (2019). Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. adv. Vet. Anim. Res., 6(3): 409-415. https://doi.org/10.5455/javar.2019.f361
Gouda M, Bekhit AED, Tang Y, Huang Y, Huang L, He Y, Li X (2021). Recent innovations of ultrasound green technology in herbal phytochemistry: A review. Ultrason. Sonochem., 73: 1-15. https://doi.org/10.1016/j.ultsonch.2021.105538
Isroli I, Yudiarti T, Widiastuti E, Sugiharto S (2017). Effect of decocted turmeric on performance, hematological parameters and carcass traits of broiler chickens. J. Indones. Trop. Anim. Agric., 42(4): 263-269. https://doi.org/10.14710/jitaa.42.4.263-269
Kieliszek M, Pobiega K, Piwowarek K, Kot AM (2021). Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules, 26(7): 1-15. https://doi.org/10.3390/molecules26071858
Kikusato M (2021). Phytobiotics to improve health and production of broiler chickens: functions beyond the antioxidant activity. Anim. Biosci., 34(3): 345-353. https://doi.org/10.5713/ab.20.0842
Kikusato M, Toyomizu M (2023). Mechanisms underlying the effects of heat stress on intestinal integrity, inflammation, and microbiota in chickens. J. Poult. Sci., 60(2): 1-14. https://doi.org/10.2141/jpsa.2023021
Klojdová I, Milota T, Smetanová J and Stathopoulos C (2023) Encapsulation: A Strategy to Deliver Therapeutics and Bioactive compounds?. Pharmaceuticals. 16(3): 1-9.
Kpomasse CC, Oke OE, Houndonougbo FM, Tona K (2021). Broiler production challenges in the tropics: A review. Vet. Med. Sci., 7(3): 831-842. https://doi.org/10.1002/vms3.435
Krismiyanto L, Suthama N, Mulyono M, Ningrum MA (2022). Feeding of glucomannans and anthocyanins combination in the containing microparticle protein on fat digestibility and fat deposition on broiler chicken. J. Ilmiah Biol., 11(2): 190-200. https://doi.org/10.26877/bioma.v11i2.11533
Liu Y, Wang J and Wu C (2022). Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front. Nutr., 8: 1-14. https://doi.org/10.3389/fnut.2021.634897
Mangisah I, Krismiyanto L, Ismadi VDYB, Mulyono M, Sukamto B, Wahyono F, Suthama N (2020). Studies on intestinal ecology and growth performance of Tegal ducks fed with Lactobacillus casei and porang (Amorphophallus oncophyllus) tuber extract. livestock res. rural dev., 32(8).
Mangisah I, Yunianto VD, Sumarsih S, Sugiharto S (2021). Supplementation of garlic powder and Lactobacillus casei to improve nutrient digestibility, physiological conditions, and performance of broiler during starter phase. Journal of the Indonesian Trop. Anim. Agric., 46(4): 336-346. https://doi.org/10.14710/jitaa.46.4.336-346
McDonald, PRA, Edwards and JFH Greenhalgh. 1977. Animal Nutrition. 4th Ed. Longman. Hong Kong.
Mohammed A, Mahmoud M, Murugesan R, Cheng HW (2021). Effect of a synbiotic supplement on fear response and memory assessment of broiler chickens subjected to heat stress. Animals., 11(2): 1-15. https://doi.org/10.3390/ani11020427
McDonald, PRA, Edwards and JFH Greenhalgh (1977). Animal Nutrition. 4th Ed. Longman. Hong Kong.
Natsir MH, Sjofjan O, Widodo E, Ardiansah I, Widyastuti ES (2019). Effect of either non-encapsulated or encapsulated acidifier-phytobiotic-probiotic on performance, intestinal characteristics and intestinal microflora of local hybrid ducks. Livestock Res. Rural Dev., 31(1): 1-5.
NRC (1994). Nutrient Requirements of Poultry: Ninth Revised Edition. National Acad. Press. Wash. D.C.,
Pearlin BV, Muthuvel S, Govidasamy P, Villavan M, Alagawany M, Ragab FM, Kuldeep D, Gopi M (2020). Role of acidifiers in livestock nutrition and health: A review. J. anim. Physiol. Anim. Nutr., 104(2): 558-569. https://doi.org/10.1111/jpn.13282
Prakasita VC, Asmara W, Widyarini S, Wahyuni AETH (2019). Combinations of herbs and probiotics as an alternative growth promoter: an in vitro study. Vet. World, 12(4): 614-620. https://doi.org/10.14202/vetworld.2019.614-620
Sapsuha Y, Suprijatna E, Kismiati S, Sugiharto S (2021). Combination of probiotic and phythobiotic as an alternative for antibiotic growth promoter for broilers chickens-a review. Livestock Res. Rural Dev., 33(4).
Sapsuha Y, Hasan S, Nur A (2023). The effect of symbiotic supplementation of nutmeg flesh extract (Myristica fragrans Houtt) and Lactobacillus plantarum on microbial and intestinal morphology of broiler chickens. Livestock Res. Rural Dev., 35(1).
Saracila M, Panaite TD, Papuc CP, Criste RD (2021). Heat stress in broiler chickens and the effect of dietary polyphenols, with special reference to willow (Salix spp.) bark supplements—A Review. Antioxidants, 10(5), 1-29. https://doi.org/10.3390/antiox10050686
Srivastava RP, Kumar S, Singh L, Madhukar M, Singh N, Saxena G, Pandey S, Singht A, Dvkota HP, Verma PC, Shiva S, Malik S, Rustagi S (2023). Major phenolic compounds, antioxidant, antimicrobial, and cytotoxic activities of Selinum carvifolia (L.) collected from different altitudes in India. Front. Nutr., 10: 1-16. https://doi.org/10.3389/fnut.2023.1180225
Sunu P, Sunarti D, Mahfudz LD, Yunianto VD (2021). Effect of synbiotic from Allium sativum and Lactobacillus acidophilus on hematological indices, antioxidative status and intestinal ecology of broiler chicken. J. Saudi Soc. Agric. Sci., 20(2): 103-110. https://doi.org/10.1016/j.jssas.2020.12.005
Sugiharto S, Yudiarti, T, Isroli I, Widiastuti E, Kusumanti E (2017). Dietary supplementation of probiotics in poultry exposed to heat stress–a review. Ann. Anim. Sci., 17(3): 591-604. https://doi.org/10.1515/aoas-2016-0062
Sugiharto S, Yudiarti T, Isroli I, Widiastuti E, Putra FD (2017). Intestinal microbial ecology and hematological parameters of broiler fed cassava waste pulp fermented with Acremonium charticola. Vet. World, 10(3): 324-330. https://doi.org/10.14202/vetworld.2017.324-330
Sugiharto S and Ayasan T (2022). Encapsulation as a way to improve the phytogenic effects of herbal additives in broilers. Ann. Anim. Sci., 23(1): 53-68. https://doi.org/10.2478/aoas-2022-0045
Vimon S, Angkanaporn K, Nuengjamnong C (2023). Microencapsulation of Lactobacillus plantarum MB001 and its probiotic effect on growth performance, cecal microbiome and gut integrity of broiler chickens in a tropical climate. Anim. Biosci., 36(8): 1252 - 1262. https://doi.org/10.5713/ab.22.0426
Wang J, Yao L, Su J, Fan R, Zheng J, Han Y (2023). Effects of Lactobacillus plantarum and its fermentation products on growth performance, immune function, intestinal pH, and cecal microorganisms of Lingnan yellow chicken. Poult. Sci., 102(6): 1-8. https://doi.org/10.1016/j.psj.2023.102610
Yuanita I, Sunarti D, Wahyuni HI, Suthama N (2019). Feeding Dayak onion (Eleutherine palmifolia) extract and Lactobacillus acidophilus mixture on blood biochemicals, meat quality characteristics and growth performance in broiler chickens. Livestock Res. Rural Dev., 31(9).
To share on other social networks, click on any share button. What are these?






