African Swine Fever Virus Transmission Cycle in Nigeria: Assessment of Domestic Pig-Soft Tick Contact through Detection of Antibodies against Ornithodoros moubata Salivary Antigen TSGP1
African Swine Fever Virus Transmission Cycle in Nigeria: Assessment of Domestic Pig-Soft Tick Contact through Detection of Antibodies against Ornithodoros moubata Salivary Antigen TSGP1
Pam D Luka1*, Frank N Mwiine2, Bitrus Yakubu1, Joseph Erume2, Ricardo Pérez-Sánchez3, Hermann Unger4 and David Shamaki1
1National Veterinary Research Institute, Vom, Nigeria; 2Makerere University, Kampala, Uganda; 3Parasitología, IRNASA (CSIC), Cordel de Merinas, 40-52, 37008 Salamanca, Spain; 4Animal Production and Health Laboratory of the joint IAEA/FAO division, Seibersdorf, Austria.
Abstract | African swine fever (ASF) is a contagious viral disease of pigs with severe consequences and a growing transboundary potential. The disease is caused by ASF virus (ASFV), the only DNA virus with an arthropod vector of the genus Ornithodoros, involved in its transmission. Tick involvement has been reported in Europe, East and Southern Africa to be part of ASFV transmission cycles. Ticks in an ASFV infected area have been reported to influence the emergence of genetic variation and long-term persistence of the virus in an area. Since the first report of ASF in Nigeria, periodic outbreaks have continued to occur among domestic pigs with a few incidences in wild pigs. However, to investigate the risk of a tick-domestic pig transmission cycle in Nigeria, 3,288 serum samples were collected from domestic pigs from 10 states and analysed for anti-tick antibodies using the recombinant TSGP1 indirect ELISA. Of the samples analysed, 13.4% (442/3,288) showed moderate to high reactivity, suggestive of domestic pigs’ exposure to tick-bite. Antibody reactivity’s were found in eight of the 10 states studied and all the states have favourable climatic conditions for soft ticks’ survival and national parks harbouring wild swine (warthogs) able to maintain the developmental cycle of these ticks. This provides some evidence of interaction between the host and the vector within the Nigeria’s ecosystem. This finding provides a guide for further studies to establish their role in the epidemiology of ASF and additional effort towards understanding the ecology of Ornithodoros ticks in Nigeria.
Editor | Muhammad Abubakar, National Veterinary Laboratories, Park Road, Islamabad, Pakistan.
Received | January 13, 2017; Accepted | February 14, 2017; Published | February 18, 2017
*Correspondence | Dr. Pam Luka, National Veterinary Research Institute, Vom, Nigeria; Email: [email protected]
Citation | D. Pam Luka, P., F.N Mwiine, B. Yakubu, J. Erume, R. Pérez-Sánchez, H. Unger and D. Shamaki. 2017. African swine fever virus transmission cycle in Nigeria: Assessment of domestic pig-soft tick contact through detection of antibodies against Ornithodoros moubata salivary antigen TSGP1. Veterinary Sciences: Research and Reviews. 3(1): 6-12.
DOI | http://dx.doi.org/10.17582/journal.vsrr/2017.3.1.6.12
Introduction
African swine fever (ASF) was first observed in Nigeria in 1997 with annual outbreaks reported across the country. It was first reported in Kenya in 1921 and has been described as a highly infectious and contagious disease of domestic pigs caused by African swine fever virus (ASFV) (Tulman et al., 2009). The disease occurs in both domestic and wild pigs throughout the sub-Saharan Africa and transmitted through Ornithodoros ticks bite. Mortality can be as high as 100% in a naïve population resulting in huge tangible and intangible economic losses and socio-economic impact on production, trade and food security. The virus is considered as a lone member of the family Asfarviridae and genus Asfivirus (Dixon et al., 2000). The virus can be transmitted through an arthropod vector, a soft tick of the Ornithodoros moubata complex that is widely distributed among ASF infected countries and plays a role in the persistence and emergence of new genotypes within an infected area/country thereby complicating eradication programmes (Manzano-Román et al., 2012).
After replication within the argasid tick, the virus gets transmitted to wild pigs (warthogs) through a blood meal bite, but without producing clinical disease in these hosts. The involvement of ticks and wild pigs in this ancient sylvatic cycle as observed in East and Southern Africa has allowed for the transmission and maintenance for the virus for a long-time within a given environment and subsequently the emergence of several (Oleaga-Pérez et al., 1990; Penrith, 2009). Likewise, a domestic pig-tick cycle has additionally been depicted in some territories of East and Southern Africa without the inclusion of warthog (Penrith et al., 2004). However, transmission of the virus through direct contact (domestic pig to pig) is the most established form of transmission in the entire West African region because the sylvatic cycle is yet to be demonstrated (Costard et al., 2009).
A number of missing links exist in Nigeria on the conceivable part of ticks and wild pigs in the transmission of ASFV. Although the virus has been detected once in red river hog (Phacocoerus africanus) and bush pigs (Potamochoerus porcus), respectively from different regions of the country (Luther et al., 2007; Owolodun et al., 2010b), the presence of ticks and/or their serological evidence are yet to be demonstrated. Nevertheless, once the virus is introduced into the domestic pig population, vector transmission would no longer be a requirement. ASF is endemic in Nigeria and the common source of infection has been attributed to domestic pig-pig transmission without tick or wild suid involvement (Fasina et al., 2010; Owolodun et al., 2010a).
The presence of Ornithodoros moubata in Nigeria (Manu, 2012) needs to be further investigated. For the presence of sylvatic cycle will have a tremendous impact on the transmission and long-term maintenance of the ASFV in the country. Given the favourable agro-ecological zones (Manzano-Román et al., 2012), wild pigs and a varied pig production systems ranging from extensive free range to intensive ones that favours the survival of the ticks (Owolodun et al., 2010b; Ayas et al., 2016).
In this scenario, infected ticks will be the link between wild suids and domestic pigs (Quembo et al., 2016), and their involvement in the transmission of ASFV would greatly complicate the implementation of the necessary control measures in Nigeria. Thus, for designing an adequate and comprehensive control strategy, the presence or absence of ticks needs to be investigated.
Within the 24 Nigerian states so far affected by ASF, virus isolation has always been from domestic pigs, with two instances of ASFV detection from warthog and bush pigs from Adamawa, Bauchi and Plateau states. To our knowledge, there has not been any serological evidence, obtained from scientific data, of tick involvement in the transmission of ASFV in Nigeria.
The aim of this study was to indirectly investigate the presence and possible involvement of Ornithodoros moubata soft ticks in the ASF transmission in Nigeria. For this reason, 3,288 porcine serum samples from 10 pig producing states in Nigeria were screened for antibodies against tick saliva antigen using the recombinant TSGP1 indirect ELISA (Díaz-Martín et al., 2011).
Materials and Methods
Study Area
Nigeria is a country in Africa that lies within latitude 4o-14o N and longitude 2o-15o E with a population of 170 million covering approximately 923,763 km2 of land area. The country is divided into 36 states and the Federal Capital Territory Abuja (Figure 1). Each state is divided into local government areas (LGAs) which are the smallest administrative units. Pig production is primarily practiced in the North central and southern states but not a common practice within the northern states due to religious biases. Ten pig producing states were randomly selected from the 23 including those with/without national parks or pig markets. Stratified random sampling within cluster stratum taking into consideration infected states, local government areas (LGAs), states and areas with previous reports of ASF outbreaks were sampled.
Sample Collection
A total of 3,288 sera was collected from 330 farms across the 10 states were selected randomly between September 2012 – August 2014 (Table 1). Samples were collected from pigs managed under intensive, semi-intensive and extensive husbandry system. On average the pigs sampled were between ages 1.5 months – 4 years. Most blood samples were collected via venous puncture using sterile vacutainer tubes and needles (Venoject, UK), and some samples were collected directly from the slaughter slab. All sera collected were transported on ice to the Regional Laboratory for Avian Influenza and other Transboundary Animal Diseases (RLATAD) of the National Veterinary Research Institute (NVRI), Vom, Nigeria and stored at -20oC until tested.
Anti-tick Salivary Antigen ELISA
The presence of antibodies against the salivary proteins of Ornithodoros spp. ticks in the serum samples was assessed using the ELISA developed by Díaz-Martín et al. (2011), which is based on a recombinant salivary lipocalin protein (rtTSGP1) of O. moubata complex. Previous reports had demonstrated 99.4% specificity and 100% sensitivity of the assay and its ability to detect tick bite antibodies 3 months’ post exposure (Díaz-Martín et al., 2011).
ELISA positive and negative controls were obtained from Parasitología, IRNASA (CSIC), Salamanca, Spain and carried out according to the Laboratory recommendations. Briefly, 100 ng/well ELISA antigen (rtTSGP1) was diluted in coating buffer (pH 9.6) coated and 100 µl /well used to charge microtiter plates following overnight incubation at 4 ºC. Subsequently, the plates were washed three times with wash buffer (0.05% Tween 20 in PBS) and coated with 200 µl/well of 1% BSA in PBS at 37 ºC for 1 h. This was followed by another washing and 100 µl/well of pre-diluted sera (1/300) was added in duplicate and incubated at 37 ºC for 1 h. Peroxidase-labelled anti-pig IgG (Sigma) was added at 1/10,000 dilution in TPBS and incubated. After 1 h incubation and 3 washings, 100 µl of chromogen/substrate was added and the reaction stopped with 100 µl stop solution (H2SO4, 3N) to all the wells. Optical density (OD) was taken at 492nm using an ELISA reader, Immunoskan BDSL (Thermo Lab. System, Finland). Each serum OD was converted to serological index (SI) using the formula:
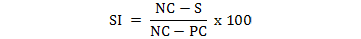
Where, NC and PC represent the negative and positive controls, respectively, and S stands for each sample serum. After that, the sample to positive control ratio (SP ratio, SP) was calculated for each sample and classified according to the matrix described by Pietschmann et al. (2016) (Table 2).
Table 1: Sampling: characteristics of sampled States and number of sera collected
| State | No. of sera collected | Very high probability (+++) positive (%) | Characteristics of states where samples were collected |
| Adamawa | 342 | 19 (5.56) | Bordering Cameroon at the mid-north & has Gashaka Gumti National Park |
| Benue | 452 | 18 (3.98) | High pig producing area |
| Cross River | 177 | 14 (7.91) | Bordering Cameroon in the south and has the Cross River Park |
| Delta | 227 | 10 (4.41) | Pig farming & pork consuming area neighboring Edo with Okumu National park |
| Enugu | 137 | 0 | Eastern heartland |
| Kaduna | 146 | 14 (9.59) | Pig producing and major pig market in the country |
| Lagos | 337 | 0 | Borders with Benin Republic to the west |
| Ogun | 355 | 0 | Borders Benin Republic & neighboring Oyo state with Old Oyo National park |
| Plateau | 568 | 2 (2.11) | High pig producing state |
| Taraba | 547 | 12 (2.19) | Bordering Cameroon at the mid-north & has Gashaka Gumti National Park |
| Total | 3288 |
89 (2.7%) |
Table 2: Classification matrix according to sample to positive ration (SP)
| SP | Category | Definition |
| <10 | 0 | Negligible probability of tick presence |
| 11-30 | + | Medium probability of tick presence |
| 31-50 | ++ | High probability of tick presence |
| >50 | +++ |
Very high probability of tick presence |
Tick Sampling
Ticks investigation was carried out in some areas where samples were serologically reactive during the dry season (October – December, 2016) when tick activities are predicted to be high although during that same time pigs are allowed to roam freely. These tick are found usually underground in cracks, crevices and holes, being most commonly found in dry-stone walls of traditional pig housing (Manzano-Román et al., 2012; Boinas et al., 2014). Pens or epidemiological units with characteristic stone construction which provide adequate habitation for Ornithodoros were targeted for tick collection (Figure 2A). Dry ice as a source of carbon dioxide were placed inside pens (Figure 2B) overnight as described by Caiado et al. (1990).
Results
From the total number of samples analysed (Table 3), our findings showed that 86.6% (2846/3288) serum samples fell under category 0 (negligible probability of the presence of ticks). Among those with medium probability of tick presence (+), only 252 samples (7.7%) showed reactivity. Another 101 sample, accounting for 3.1% were grouped under ++ (high probability of tick presence) while 89 samples (2.7%) were placed into the +++ (very high probability of tick presence). A total of 89 samples were obtained under category +++, a total of 19 (5.56%), 18 (3.98%), 14 (7.91%), 10 (4.41%), 14 (9.59%), 2 (2.11%) and 12 (2.19%) sera were from Adamawa, Benue, Cross River, Delta, Kaduna, Plateau and Taraba states, respectively (Table 3). None was reported from Enugu, Ogun and Lagos states from this category. In the category ++, positive samples were found in Adamawa (n = 6), Benue (n = 16), Cross Rover (n = 20), Delta (n = 2), Kaduna (n = 16), Lagos (n = 5), Plateau (n = 24) and Taraba (n = 12) (Table 3).
Interestingly, no reactivity was observed in Enugu and Ogun. The above result showed spatial clustering across the states for categories +++ and ++.
Table 3: Classification of sera according to their state of origin and reactivity to rtTSGP1
| State | Total number of sample | 0 | + | ++ | +++ |
| Adamawa | 342 | 281 | 36 | 6 | 19 |
| Benue | 452 | 375 | 43 | 16 | 18 |
| Cross River | 177 | 109 | 34 | 20 | 14 |
| Delta | 227 | 186 | 29 | 2 | 10 |
| Enugu | 137 | 137 | 0 | 0 | 0 |
| Kaduna | 146 | 89 | 27 | 16 | 14 |
| Lagos | 337 | 322 | 10 | 5 | 0 |
| Ogun | 355 | 355 | 0 | 0 | 0 |
| Plateau | 568 | 487 | 55 | 24 | 2 |
| Taraba | 547 | 505 | 18 | 12 | 12 |
| Total | 3288 | 2846 | 252 | 101 |
89 |
Discussion
From the first report of African swine fever in Nigeria to date, the disease has continued to spread into new areas within the country with the emergence of newer variants (El-Hicheri, 1998; Luka et al., 2016; Ayas et al., 2016). Previous works by Luther et al. (2007) and Owolodun et al. (2010b) reported single incidences each of ASFV genome in baby warthog from a forest in Adamawa and a bush-pig from Plateau states, respectively. This findings, were suggestive of the presence of a sylvatic cycle in Nigeria. However, no investigation has been carried out to establish the presence and possible role played by ticks (Ornithodoros spp.) in the transmission of ASF between wild suids and domestic pigs in Nigeria (Jori et al., 2013). This is the first study to reveal domestic pigs’ exposure to tick bite as a possible mode in the transmission of ASF in Nigeria.
From the sera samples analysed in this study, 13.44% of them showed medium to high positive reactivity indicating domestic pigs’ exposure to tick bite in 8 of the 10 states sampled. Given the specificity (90%) of the test and the spatial clustering of the positive sera across different geographical zones with suitable climatic conditions for tick survival (mean annual temperatures in the range of 22 to 28oC) (Pietschmann et al., 2016) lend support to the results of the serology. Although direct search for ticks in some of study states did not yield result possible due to several humans, environmental and climatic factors. Albeit, sampling and collection of ticks is always a challenge (Pérez de León et al., 2015). Nevertheless, our serological findings of antibody reactivity is suggestive of the presence of Ornithodoros moubata complex ticks in some of the sampled areas. Our findings are supported by the observations of Bunza et al. (2008), who reported the presence of O. moubata (14.3%) and Ornithodoros savignyi (7.1%), respectively, within the live bird’s market in Sokoto. Similarly, Manu (2012), also reported the presence of soft ticks from stables, resting shades and animal markets in relationship to human borreliosis in Maiduguri, Borno state.
As already mentioned, the detection of ASFV genome in warthogs caught in the forest of Adamawa (Luther et al., 2007) and bush pig from Plateau state (Owolodun et al., 2010b). Although, a single occurrence was suggestive of the involvement of wild pigs in the transmission of ASFV in those states. The current finding of pig sera with high and very high reactivity to tick salivary antigen in both states is suggestive of the presence of the required vector for this transmission cycle.
Again, both the wild pig-soft tick and domestic pig-soft tick transmission cycles of ASFV in Nigeria are likely. This is in agreement with the observations of Vial et al. (2007) in Senegal (West Africa), who reported that Ornithodoros sonrai ticks as a potential vector in transmission of ASF. Interestingly, our preliminary survey for ticks was not successful but serves as a guide to tick surveillance and ecological studies.
Our study is the first to assess the presence of antibodies to O. moubata salivary antigen in domestic pigs in Nigeria and its distribution across some pig producing states. The possible role of soft ticks in ASF persistence in Nigeria domestic pig’s populations is very likely, and future control and prevention measures should take that into consideration. Further application of serology in a wider tick surveillance programmes together with direct field search for ticks could help our understanding of the dynamics of ASFV in Nigeria and other West African countries.
Acknowledgements
The authors appreciate Dan L. Rock and the technical staff of Biotechnology Division of the NVRI, Vom for their support. This study was jointly funded by National Veterinary Research Institute, Vom, Plateau State, Nigeria and the IAEA Research Contract No: 18347/R0.
Conflict of Interest
The authors declare no conflicting interest.
Authors’ Contributions
LPD, MFN, YB and EJ conceived and designed the study. PR, UH and DS provided technical support for the study. LPD, YB and PR analysed and read the result. LPD, MFN, EJ and PR drafted the manuscript and all authors read and approved the final manuscript for submission.
References
- • Ayas, S.A., Bot, C.J., Jambol, A.R., Luka, P.D. Molecular detection of African swine fever virus in apparently healthy domestic pigs in Nasarawa state, Nigeria. Sokoto Journal of Veterinary Sciences, 2016; 14(3): 26-31. https://doi.org/10.4314/sokjvs.v14i3.4
- • Boinas, F., Ribeiro, R., Madeira, S., Palma, M., de Carvalho, I.L., Nuncio, S., Wilson, A.J. The medical and veterinary role of Ornithodoros erraticus complex ticks (Acari: Ixodida) on the Iberian Peninsula. Journal of Vector Ecology, 2014; 39: 238-248. https://doi.org/10.1111/jvec.12098
- • Bunza, M.D.A., Yahaya, M.M., Muhammad, A.S., Saidu, A.R. A survey on tick species infesting domestic birds sold at Sokoto central market, Nigeria. Sokoto Journal of Veterinary Sciences, 2008; 7: 52-54.
- • Caiado, J.M., Boinas, F.S., Melo, M.A., Louza, A.C. The use of carbon dioxide insect traps for the collection of Ornithodoros erraticus on African swine fever-infected farms. Preventive Veterinary Medicine, 1990; 8: 55-59. https://doi.org/10.1016/0167-5877(90)90022-A
- • Costard, S., Wieland, B., de Glanville, W., Jori, F., Rowlands, R., Vosloo, W., Roger, F., Pfeiffer, D.U., Dixon. L.K. African swine fever: how can global spread be prevented? Philosophical Transactions of the Royal Society B, 2009; 364: 2683-2696. https://doi.org/10.1098/rstb.2009.0098
- • Díaz-Martín, V., Manzano-Román, R., Siles-Lucas, M., Oleaga, A., Pérez-Sánchez, R. Cloning, characterization and diagnostic performance of the salivary lipocalin protein TSGP1 from Ornithodoros moubata. Veterinary Parasitology, 2011; 178: 163-172. https://doi.org/10.1016/j.vetpar.2010.12.014
- • Dixon, L.K., Costa, J.V., Escribano, J.M., Rock, D.L., Vinuela, E., Wilkinson, P.J. 2000. Family Asfarviridae. In: Van Regenmortel, M.H.V., Fauquet, C.M., Bishop, D.H.L., Carestens, E.B., Estes, M.K., Lemon, S.M., Maniloff, J., Mayo, M.A., McGeoch, D.J., Pringle, C.R., Wickner, R.B.F.A., Murphy, C.M., Fauquet, D.H.L., Bishop, S.A., Ghabrial, A.W., Jarvis, G.P., Martelli, M.D. (eds), Virus Taxonomy: Seventh Report of the International Committee on Taxonomy of Viruses, 159–165. Summers Academic Press, San Diego.
- • El-Hicheri, K. 1998. Emergency assistance on control and eradication of an outbreak of African swine fever in Western Nigeria. Report of the FAO Consultancy Mission to Nigeria. TCP/NIR/7822(E). FAO, Rome, December, 1998.
- • Fasina, F.O., Shamaki, D., Makinde, A.A., Lombin, L.H., Lazarus, D.D., Rufai, S.A., Adamu, S.S., Agom, D., Pelayo, V., Soler, A., Simon, A., Adedeji, A.J., Yakubu, B., Mantip, S., Benshak, J.A., Okeke, I., Anagor, P., Mandeng, D.C., Akanbi, B.O., Ajibade, A.A., Faramade, I., Kazeem, M.M., Enurah, L.U., Bishop, R., Anchuelo, R., Martin, J.H., Gallardo, C. Surveillance for African swine fever in Nigeria, 2006-2009. Transboundary Emerging Disease, 2010; 57: 244-253. https://doi.org/10.1111/j.1865-1682.2010.01142.x
- • Jori, F., Vial, L., Penrith, M.L., Pérez-Sánchez, R., Etter, E., Albina, E., Michaud, V., Roger, F. Review of the sylvatic cycle of African swine fever in Sub-Saharan Africa and the Indian Ocean. Virus Research, 2013; 173: 212-227. https://doi.org/10.1016/j.virusres.2012.10.005
- • Luka, P.D., Achenbach, J.E., Mwiine, F.N., Lamien, C.E., Shamaki, D., Unger, H., Erume, J. Genetic Characterization of circulating African swine fever viruses in Nigeria (2007–2015) Transboundary and Emerging Diseases, 2016. https://doi:10.1111/tbed.12553
- • Luther, N.J., Udeama, P.G., Majiyagbe, K.A., Shamaki, D., Antiabong, J.F., Bitrus, Y., Nwosuh, C.I., Owolodun, O.A. Polymerase chain reaction (PCR) detection of the genome of African swine fever virus (ASFV) from natural infection in a Nigerian baby warthog (Phacochoereus aethiopicus). Nigerian Veterinary Journal, 2007; 28: 63-67.
- • Manu, J.I. 2012. Socio-demographic features and risk factors associated with suspected cases of tick-borne relapsing fever in northern Borno state, Nigeria. MPH thesis submitted to Ahmadu Bello University, Zaria, Nigeria.
- • Manzano-Román, R., Díaz-Martín, V., Fuente, J., Pérez-Sánchez, R. 2012. Soft ticks as pathogen vectors: Distribution, surveillance and control. Parasitology, Dr. Mohammad Manjur Shah (Ed.), ISBN: 978-953-51-0149-9, InTech, Available from: http://www.intechopen.com/books/parasitology/softticks-as-pathogen-vectors-distribution-surveillance-and control.
- • Oleaga-Pérez, A., Pérez-Sánchez, R., Encinas-Grandes, A. Distribution and Biology of Ornithodoros erraticus in the African Swine Fever Enzootic Area of Spain. Veterinary Records, 1990; 126: 32-37.
- • Quembo, C.J., Jori, F., Pérez-Sánchez, R., Heath, L., Vosloo, W. Investigation into the epidemiology of African swine fever virus at the wildlife–domestic interface of the Gorongosa National Park, Central Mozambique. Transboundary Emerging Disease, 2016; 63: 443-451. https://doi.org/10.1111/tbed.12289
- • Owolodun, A.O., Bastos, A.D.S., Antiabong, J.F., Ogedengbe, M.E., Ekong, P.S., Yakubu, B. Molecular characterization of African swine fever virus variants and reaffirms CVR epidemiological utility. Virus Genes, 2010a; 41: 361-368. https://doi.org/10.1007/s11262-009-0444-0
- • Owolodun, A.O., Yakubu, B., Antiabong, J.F., Ogedengbe, M.E., Luka, P.D., John Audu, B., Ekong, P.S, Shamaki, D. Spatio-temporal dynamics of African swine fever outbreaks in Nigeria, 2002-2007. Transboundary Emerging Disease, 2010b; 57: 330-339. https://doi.org/10.1111/j.1865-1682.2010.01153.x
- • Penrith, M.L. African swine fever. Onderstepoort Journal of Veterinary Research, 2009; 76: 91-95. https://doi.org/10.4102/ojvr.v76i1.70
- • Penrith, M.L., Thomson, G.R., Bastos, A.D.S. 2004. African swine fever. In: Coetzer, J.A.W, Tustin, R.C. (Eds). Infectious diseases of livestock with special reference to Southern Africa. 2nd ed. Oxford University Press, Cape Town, pp. 1087-1119.
- • Pietschmann, J., Mur, L., Blome, S., Beer, M., Pérez-Sánchez, R., Oleaga, A., Sánchez-Vizcaíno, J.M. African swine fever virus transmission cycles in Central Europe: Evaluation of wild boar-soft tick contacts through detection of antibodies against Ornithodoros erraticus saliva antigen. BMC Veterinary Research, 2016; 12: 1. https://doi.org/10.1186/s12917-015-0629-9
- • Pérez de León, A., Showler, A., Stegniy, B.T., Kucheryavenko, R.O., Kucheryavenko, V.V., Gerilovych, A.P., Filatov, S.V., Li, A., Teel, P., McVey, S. Soft tick sampling and collection. Journal of Veterinary Medicine and Biotechnology and Biosafety, 2015; 1: 1-11. Tulman, E.R., Delhon, G.A., Ku, B.K., Rock, D.L. African swine fever virus. Current Topics in Microbiology and Immunology, 2009; 328: 43-87. https://doi.org/10.1007/978-3-540-68618-7_2
- • Vial, L., Wieland, B., Jori, F., Etter, E., Dixon, L., Roger, F. African swine fever virus in soft ticks, Senegal. Emerging Infectious Disease, 2007; 13: 1928-1931. https://doi.org/10.3201/eid1312.071022
To share on other social networks, click on any share button. What are these?






