Ameliorating Potential of Quercetin on Liver Function, Genotoxicity and Oxidative Damage Induced by 2,3,7,8- Tetrachlorodibenzo-P-Dioxin in Liver of Male Rats
Ameliorating Potential of Quercetin on Liver Function, Genotoxicity and Oxidative Damage Induced by 2,3,7,8- Tetrachlorodibenzo-P-Dioxin in Liver of Male Rats
Saman Muhsin Abdulkareem* and Nadir Mustafa Nanakali
Department of Biology, College of Education, Salahaddin University-Erbil, Iraq
ABSTRACT
In this study, the protective effects of antioxidant quercetin(QCT) were studied on indices of oxidative stress, liver enzymes activity and the expression of cytochrome P450 1A1 (CYP1A1) against hepatotoxicity induced by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) in male rat. Thirty adult male Wistar rats randomly divided into five equal groups. TCDD and QCT were orally administered at the dose of 10µg/kg /day and 20 mg/kg/day, respectively by gavage dissolved in corn oil for 90 days. At the end of the study, animals were sacrificed and their liver were removed for biochemical analysis including the level of oxidative stress biomarkers, liver enzymes, and the expression of CYP1A1 gene. The results of this study indicated that exposure to TCDD (10µg/kg/day) could significantly increase liver oxidative stress biomarkers and serum liver enzymes (p<0.05). While, pre and post treatment of QCT (20 mg/kg/day) had protective effects on hepatotoxicity induced by TCDD and could significantly decrease these factors (p<0.05). Furthermore, there is no difference between the pre and post treatment of QCT (p>0.05). In addition to, the results showed that prolonged exposure to TCDD causes mutation in CYP1A1 gene and increases the expression of this gene in liver cells, while these effects were not observed in the pre and post treatment of QCT in rats treated with TCDD. The results showed that exposure to TCDD for 90 consecutive days could cause liver damage by oxidative stress, genotoxicity effect and alteration in the expression of CYP1A1 gene, and quercetin was able to cure these damages.
Article Information
Received 28 May 2019
Revised 13 July 2019
Accepted 24 July 2019
Available online 17 January 2020
Authors’ Contribution
SMA did the acquisition of data, analysed and interpreted the data. NMN presented the concept and designed the study. NMN and SMA drafted the manuscript.
Key words
TCDD, Quercetin, CYP1A1, Liver, Oxidative stress, Mutation.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190708100752
* Corresponding author: [email protected]
0030-9923/2020/0002-0535 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) is a polychlorinated dibenzo-p-dioxin with the chemical formula C12H4Cl4O2 which has a long half-life of 5-10 years in human beings as a result of its high lipophilicity, and little or no metabolism(Sorg et al., 2009; Beischlag et al., 2008). It is usually formed as a side product in organic synthesis and burning of organic materials (Brown et al., 2005). Prolong exposure to TCDD may result in a wide variety of adverse biological effects, such as reproductive and developmental defects, teratogenicity, carcinogenicity, immunotoxicity, hepatotoxicity, cardiotoxicity, dermatological disease, endocrine disruption, and numerous other biochemical alterations (Bruner-Tran et al., 2017; Ngo et al., 2006; Patrizi and Siciliani de Cumis, 2018; Van den Berg et al., 2006; Chang et al., 2014). In 1997, World Health Organization’s International Agency of Research on Cancer (IARC) was classified TCDD in group 1. Then in 2009, the institute confirmed that TCDD is a human carcinogen and is associated to the increased mortality from all types of cancers (Van den Berg et al., 2006; Chang et al., 2014; Boffetta et al., 2011; El-Gendy and El-Kadi, 2013). The biological adverse effects of TCDD were performed through aryl hydrocarbon receptor (AhR)-mediated signaling pathways (Larigot et al., 2018). AhR is a ligand-activated transcription factor in cytoplasm in association with Hsp90 that controls the expression of cytochrome P450(CYP)CYP1A1 in response to halogenated aromatic hydrocarbons such as TCDD (Beischlag et al., 2008; Wiest et al., 2016; Tian et al., 2003). The CYP1A1, a member of the CYP1gene family, plays an important role in the metabolism of many xenobiotic compounds (Tamaki et al., 2005). Activation of AhR by TCDD initiates the transcriptional regulation of CYP1A1 gene (Wiest et al., 2016; Tian et al., 2003). Although it is reported that TCDD like chemicals alter expression of numerous genes in liver, it is still unclear which pathways lead to major toxicities such as hepatotoxicity. Under specific condition, CYP1A1 as an enzyme catalyzes the bioactivation of compounds that can react with macromolecules, such as DNA, leading to the start of the mutagenic process (Al-Dhfyan et al., 2017; Larigot et al., 2018; Patrizi and Siciliani de Cumis, 2018). It is well established that high-dose exposure to TCDD results in oxidative stress in multiple tissues and cells (Lin et al., 2007; Reichard et al., 2006), and the oxidative stress responses are also associated with the mutagenic process (Reichard et al., 2006; Patrizi and Siciliani de Cumis, 2018). However, the mutagenic effects of TCDD on CYP1A1 genes remain unknown. In addition to, interaction of TCDD with AhR lead to increase expersion of CYP1A and CYP1B which contribute to the generation of reactive oxygen species (ROS) formation and liver tumor promotion (Larigot et al., 2018; Lin et al., 2007). In laboratory animals, exposure to TCDD lead to increase the production of ROS, lipid peroxidation and damage to DNA (Lin et al., 2007; Reichard et al., 2006). Therefore, the use of supplements and plant compounds such as quercetin to enhance the body’s antioxidant system can improve the status of oxidative stress and prevent the onset of these disorders. In agreement with this evidence, Ashida et al. (2000) reported that flavonoids such as quercetin, rutin, and luteolin are a good dietary candidate for preventing TCDD toxicity through suppressing AhR transformation (Ashida et al., 2000). It has been reported from their results that such flavonoids antagonistically inhibited AhR transformation in the rat hepatic cytosol (Ashida et al., 2000; Paganga and Rice-Evans, 1997). In addition to, over 90% of people exposure to TCDD via the food, specialy through the consumption of foods derived from animals (Weber et al., 2018), it is difficult and expensive to remove TCDD from food. Therefore, it is important to search for a food factor that offers protection from TCDD toxicity. Quercetin (3, 5, 7, 3’, 4’-pentahydroxyflavone, QCT), a yellow powder compound extracted from natural sources, has a powerful antioxidant activity in scavenges oxygen free radicals. It is found in several daily foods such as onion, grape, nuts, tea, berries, cabbage and cauliflower. Quercetin has recently been considered for antioxidant, anti-inflammatory and anti-apoptotic activities (Yao et al., 2012; Ghaffari and Hajizadeh-Moghaddam, 2018; de Pascual-Teresa et al., 2004; Alharbi et al., 2019). In animal studies were shown that QCT could reduce the liver toxicity induced by TCDD due to its antagonistic activity against AhR (Ciftci et al., 2012, 2013). Although studies showed that QCT protect the rats liver from TCDD-induced toxicity (Ciftci et al., 2013; Turkez et al., 2012), the precise role of CYP1A1 and the effects of the genetic toxicity of TCDD and protective effects of QCT on this enzyme is still unknown. Therefore, this study was designed to investigate the protective effects of QCT on oxidative stress biomarkers, liver enzymes activity, the expression of CYP1A1 and the effects of mutagenesis of TCDD-induced liver toxicity in male rats.
Materials and Methods
Chemicals
TCDD (purity>99%) and quercetin (purity>99%) were purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China), TPTZ (2, 4, 6-tripyridyltriazine) and ascorbic acid were obtained from Sigma-Aldrich Co., USA. Corn oil was obtained from local markets. All other chemicals used were obtained from the local suppliers. TCDD and QCT were dissolved in corn oil and formulated to 10µg/ml and 20mg/ml, respectively. The dose of TCDD and QCT were chosen on the basis of previous studies (Lu et al., 2011; Ciftci et al., 2012).
Ferric reducing antioxidant power assay (FRAP assay)
The antioxidant capacity of QCT was evaluated using the ferric reducing antioxidant power test (FRAP test) which was explained by Benzie and Strain (1996). Briefly, The FRAP reagent was prepared by A solution of 300mM acetate buffer (pH= 3.6), 10mM TPTZ in 40mM HCl and 2mM of FeCl3.6H2O in the proportion of 10:1:1 at 37°C. 3.995 ml of this solution was mixed with 5 μl of diluted QCT. When an intense blue color complex was formed the absorbance at 593 nm was recorded against a reagent blank (3.995 ml FRAP reagent+5 μl distilled water). Ferrous sulfate has been used as a standard at different concentrations (0-100mM) in the same condition, and ascorbic acid has been used as a reference. The FRAP value was calculated and declared as mM Fe+2 equivalent per sample of 100g or µM Fe+2 equivalent per gram sample using the Fe+2 calibration curve (Fig. 1). The following equation calculates the FRAP value for every chemical that used:
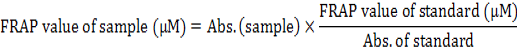
Animals
All investigations were conducted in accordance with the “Guiding Principles for the Care and Use of Research Animals” approved by Salahaddin University-Erbil with reference number: 918-761-0484. Thirty adult male Wistar rats (220±15 g) colony-bred in the Animal House Center, Salahaddin University-Erbil, Iraq were housed (six rats per cage) in the animal room under controlled lighting (12 h light: 12 h darkness) and temperature (20±2ºC) conditions and had free access to a pelleted food (formulated and made by Javaneh Khorasan Company, Iran) and tap water.
Experimental design
The effect of TCDD on the oxidative stress biomarkers, liver enzymes activity, the expression of CYP1A1 and the TCDD mutagenic effects and the role of QCT were studied by dividing the animals into five groups, each cage included six animals and was treated orally as follows: Group1, the control group (received vehicle, i.e., 1 ml/day of corn oil); Group 2, the TCDD group was orally administered TCDD (10µg/kg body weight (BW)/day); Group 3, the QCT group, rats was treated with QCT (20mg/kg BW/day); Group4, the QCT-TCDD group, TCDD (10µg/kg BW/day) was orally administered 30 min after treatment with QCT (20mg/kg BW/day) and Group 5, the TCDD-QCT group, TCDD (10µg/kg BW/day) was administrated 30 minutes before from the administration of the QCT (20mg/kg BW/day).
The TCDD, QCT and the vehicle were administered by gavage between the hour of 09:00 am and 09:30 am daily for 90 consecutive days.
Sampling and tissue preparation for biochemical analysis
The animals were sacrificed at the end of study under ether anesthesia. Whole blood was collected by heart puncture. Blood samples were drawn into blood-collecting tubes. After blood clotting, the sera were collected and stored at ±20ºC until analysis. Liver was quickly removed and weighed. The liver tissue was homogenated using an Ultra-Turrax (Janke and Kunkel IKA, Labortechnik, Germany) homogenizer at 20000 rpm/min. The resulting homogenate was centrifuged at 8000 rpm for 10 min at 4°C. The upper clear supernatants were used for the biochemical analysis.
Body weight, liver weight and hepatosomatic index
Body weight (g), liver weight (g), and hepatosomatic index (HSI) were evaluated as a measurement for the energy reserves of an animal. Body weights of the rat were measured once per week. At the end of study, HSI was calculated by using the following equation:
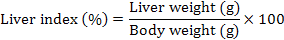
Evaluation of liver function
The levels of hepatic enzymes including alkaline phosphatase (ALP), alanine and aspartate aminotransferase (ALT and AST) as biochemical parameters were assayed for evaluating liver function. The serum ALP activity was evaluated by the method described by Wright et al. (1997). ALT and AST activities were evaluated by the method described by Huang et al. (2006).
Lipid peroxidation and antioxidant assay
Lipid peroxidation level of liver tissue was measured by determining the MDA production and the antioxidant profiles such as superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GSH-R) levels were assayed by spectrophotometric methods using the kits supplied by Beijing Solarbio Science and Technology Co., Ltd, Beijing, China.
Protein content
The total protein concentration of tissue homogenates was determined according to Lowry et al. (1951).
RNA extraction and cDNA synthesis
RNA samples were isolated from rat’s liver tissue using the extraction kit (Bioneer, ExiPrepTM Tissue total RNA kit, Korea) according to the manufacturer’s instruction. Quantification and qualification of total RNA concentration were obtained using Biophotometer (Eppendorf, Germany). Ipsogen RT Kit (Qiagen, GmbH, Hilden, Germany) was used to convert mRNA isolated to cDNA. Master-cycler pro PCR (Eppendorf, German) was used in thermal cycling processes in obtaining cDNA.
Primer design
Online primer design program (http://workbench.sdsc.edu) was employed for designing of P4501A1/Mut and P4501A1/Exp. The sequence of the primers, annealing temperature and the length of PCR products are given in Table I.
Table I.- Primer sequences, PCR product size of three targets region of P4501A1/Mut and P4501A1/Exp gene, and optimal annealing temperature.
|
Primer name / Sequence (5’ to 3’) |
Optimal annealing temp. |
PCR product size (bp) |
|
|
P4501A1/Exp |
|||
|
F: GTCACGCTCCCCTGAAGAC |
55.8°C |
236 |
|
|
R: CAGGAGCTGACACTTGGAGG |
|||
|
P4501A1/Mut1 |
|||
|
F: ATTAATCCCGGAGAGCCAGAG |
55.1°C |
865 |
|
|
F: GTGAGCCTGTTACTTGTGCC |
|||
|
P4501A1/Mut2 |
|||
|
F: AACCTATGGGAAGCCAACGA |
54.2°C |
911 |
|
|
R: GGAGACAGTATGTCGTCGCA |
|||
|
P4501A1/Mut3 |
|||
|
F: TGGTTCTGCTCCTGGTAACG |
55.3°C |
876 |
|
|
R: AGGATAACAGGTCTGCCTGC |
|||
|
GAPDH/Exp |
|||
|
F: AGTGCCAGCCTCGTCTCATA |
55.6°C |
248 |
|
|
R: GATGGTGATGGGTTTCCCGT |
|||
PCR optimization
A cDNA sample was used to carry out the gradient PCR for each primer pairs. Amplification was performed in a Master-cycler pro PCR System (Eppendorf, German). The determination of the optimum melting temperature of all primers was counted on the result of agarose gel electrophoresis. The final 25 μL PCR reaction mixture was carried out using 2.5 μL of 10× buffer Ammonium Sulfate (NH4)2SO4, 2 µL of 25 mM MgCl2, 1.5 µL of 2 mM dNTP and 0.5 µL of 5 U/μL Taq DNA polymerase, 2 μL of the cDNA template, 1 μL of 20 mM primer and 14.875 μL of ddH2O. The conditions of the gradient PCR reaction were performed with the following cycling conditions: 95°C for 5 min and 35 cycles of 95°C for 30 sec, 55–60°C for 30 sec, and 72°C for 30 sec, followed by 72°C for 2 min. Agarose gel electrophoresis was employed for primer optimization purpose. The samples were run in 2% agarose gel and stained with a compound that makes the DNA visible under UV light.
Real-time PCR
The reaction of real-time PCR has been performing based on applying the IQ5 RT-qPCR instrument (Biorad, USA). As master mix used in expression analysis, RT² SYBR Green ROX FAST Master Mix (Qiagen GmbH, Hilden, Germany) for CYP1A1 and GAPDH expression was used. Real-Time PCR master mix was prepared on ice was performed using approximately 3 μL of 50 ng cDNA template in a 25 μL reaction mixture (7.125 μL RT² SYBR Green ROX FAST Master mix, 2 μL 25 mM MgCl2, 1 μL of primer (10 mM)), and 12.875 μL RNase/DNase free water. The cDNA of each sample was added into tubes, including real-time PCR master mix. Each tube was mixed carefully, centrifuged briefly and placed on the ice again. The program saved in the IQ5 RT-qPCR instrument (Biorad, USA) was run as: enzyme activation 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 sec, primer annealing/extension at 60°C for 60 sec, melting curve at 60°C for 1 sec, and the final step at 95°C as continuous.
Nucleotide sequencing
The PCR product of the CYP1A1/Mut was separated from the agarose gel and used as a source of cDNA template for PCR amplification (Eppendorf, German). The PCR products were cleanup with ExoSAP (Thermo Fisher Scientific, USA) according to the manufacturer’s instruction. Briefly, the ExoSAP mixture consists of the sterile water, exonuclease I (10U/µl) and shrimp alkaline phosphatase (1 U/µl). The ExoSAP mixture was prepared as follows: 5µl of PCR product+2µl of Exo/SAP. The purification of cDNA template was performed in the thermocycler (Eppendorf, German) according to the conditions indicated in Table II.
Cycle sequencing reaction
The protocol of cycle sequencing is shown in Table III. The cDNA samples were placed in the thermocycler for amplification, and the program of the sequencing PCR reaction was run as: pre-denaturation 95°C for 1 min, followed by 25 cycles of denaturation at 96°C for 10 sec, primer annealing at 50°C for 5 sec, extension at 60°C for 30 min, and the final holding at 4°C as continuous.
Table II.- Conditions for PCR product cleaning up with ExoSAP.
|
Step |
Temp. |
Time (min) |
|
1. Left over primers are degraded |
37ºC |
30 |
|
2. Enzyme is degraded |
85ºC |
15 |
|
3. Hold |
4ºC |
∞ |
Table III.- The protocol of Cycle sequencing per reaction.
|
Chemical Substances |
Quantity |
|
DNA |
1µl |
|
Forward primer (0.8 µM) |
2µl |
|
5X BigDye buffer |
2µl |
|
BigDye (v3. 0) Mix |
1µl |
|
ddH2O |
4µl |
Statistical analysis
Data for this experiment were expressed as the mean ± standard deviation (SD). Analysis of results was performed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test to determine significant differences between the experimental groups using Graph Pad Prism 6 version 6.01 for Windows (Graph Pad Software, 2012). Statistical significance was set at p<0.05.
Results
Phytochemical results
The FRAP value was calculated by drawing the graph of the ferrous sulfate calibration curve at different ranges (0-100μM) (Fig. 1). The results showed that the QCT have a good antioxidant capacity compared to ascorbic acid. The FRAP value of QCT is 4906.4 μM/g Fe (II) dry weight, whereas the FRAP value of ascorbic acid is 8370.5 μM/g Fe (II) dry weight (Table IV).
Table IV.- Antioxidant activity of QCT in comparison to ascorbic acid.
|
Compound |
Absorbance |
FRAP value |
||
|
μM |
μM/mL |
μM/mg |
||
|
QCT |
1.364 |
49.064 |
490.64 |
4906.4 |
|
Ascorbic acid |
2.327 |
83.705 |
837.05 |
8370.5 |
Table V.- The mean (±SD) for liver weight (g), body weight (g) and liver index (%) in studied groups at the end of the treatment period.
|
Groups |
Body weight (g) |
Liver weight (g) |
Liver index (%) |
|
Control |
294.83 ± 7.60 |
8.15 ± 0.86 |
2.76 ± 0.23 |
|
TCDD |
244.16 ± 13.92** |
10.80 ± 0.89** |
3.14 ± 0.29 |
|
QCT |
286.83 ± 8.18## |
7.73 ± 1.14## |
2.69 ± 0.39 |
|
QCT-TCDD |
303.66 ± 8.71## |
8.59 ± 0.67## |
2.83 ± 0.24 |
|
TCDD-QCT |
320.66 ± 10.48**#$$ |
9.12 ± 0.33# |
2.84 ± 0.12 |
Results indicates as mean±SD. *, ** for p<0.01, p<0.001, respectively (vs. control group); #, ## for p<0.05, p<0.001, respectively (vs. TCDD group); $, $$ for p<0.05, p<0.001, respectively (vs. QCT group).
Body weight and liver weight
Results of mean (± SD) body weight (g), liver weight (g), and liver index (%) are presented in Table V. There are statistically significant decrease in body weight after 90 days of TCDD administration in TCDD group rats in comparison to control group rats (p<0.001). The results showed that co-treatment of QCT with TCDD in QCT-TCDD and TCDD-QCT groups reversed this effect. The results showed that administration of QCT before TCDD was more effective than administration of QCT after TCDD (QCT-TCDD: 23.90±5.25%; TCDD-QCT: 32.77±5.68%; p<0.001). The TCDD treated rats showed an insignificant (p>0.05) increase in the HSI as compared to the control rats (p>0.05). The HSI of the QCT group was insignificantly lower than other groups (p>0.05).
Liver function
In this study the level of serum liver enzymes was measured to evaluate liver function. The mean values (±SD) of AST, ALT, and ALP activities in the rat liver tissue are presented in Table VI. The results showed that the activities of AST, ALT and ALP enzymes were significantly increased in TCDD group compared to other groups (p<0.05). The activities of these enzymes were reduced significantly in QCT-TCDD (pre-treatment) and TCDD-QCT (post-treatment) rats, when compared with TCDD group (p<0.05). The results of the study showed that the levels of these enzymes had no significant difference between in pre-treatment and post-treatment of the TCDD-intoxicated groups with QCT (p>0.05). The activities of these enzymes recovered partially in both pre- and post-treated groups in comparison to TCDD group but were higher than control rats (p<0.05).
Table VI.- The mean (±SD) for the activities of liver enzymes in control and treated groups.
|
Groups |
Parameters |
||
|
AST (U/L) |
ALT (U/L) |
ALP (U/L) |
|
|
Control |
113.16 ± 15.70 |
43.83 ± 7.25 |
183.51 ± 18.12 |
|
TCDD |
243.16±17.15*** |
63.33 ±10.61** |
293.66±16.00*** |
|
QCT |
107.50±12.16## |
39.17 ± 5.15## |
171.53 ± 17.06## |
|
QCT-TCDD |
178.33 ± 17.02***##$$ |
48.67 ± 8.43# |
202.01 ± 15.84##$ |
|
TCDD-QCT |
172.83 ± 16.60***##$$ |
49.83 ± 6.01# |
237.7 ± 12.91***##$$ |
Results indicates as mean ±SD. *, **, *** for p<0.05, p<0.01, p<0.001, respectively (vs. control group); #, ## for p<0.05, p<0.001, respectively (vs. TCDD group); $, $$ for p<0.05, p<0.001, respectively (vs. QCT group).
Table VII.- The mean (±SD) for oxidative stress biomarkers in control and treated groups.
|
Groups |
Parameters |
|||
|
MDA (nmol/ mg protein) |
GSH-R (µg/ mg protein) |
SOD (U/mg protein) |
CAT (U/ mg protein) |
|
|
Control |
20.80 ± 10.21 |
116.02 ± 21.92 |
80.35 ± 14.39 |
71.91 ± 11.85 |
|
TCDD |
53.28 ± 10.62*** |
55.60 ± 18.36*** |
46.71 ± 10.28** |
36.72 ± 12.98*** |
|
QCT |
18.89 ± 12.49### |
123.69 ± 22.10### |
90.26 ± 12.54### |
77.26 ± 11.76### |
|
QCT-TCDD |
30.48 ± 12.63# |
90.23 ± 18.32 |
70.91 ± 12.83# |
61.96 ± 10.36## |
|
TCDD-QCT |
33.56 ± 12.98 |
83.72 ± 27.42$ |
65.65 ± 14.28$ |
52.02 ± 9.91*$$ |
All values are indicated as mean±SD. * p<0.05; ** p<0.01; *** p<0.001 compared to vehicle control; ≠ p<0.05; ≠≠ p<0.01; ≠≠≠ p<0.001 compared to TCDD alone; $ p<0.05; $$ p<0.01; $$$ p<0.001 compared to QCT alone.
Lipid peroxidation and antioxidant activities
The activities of antioxidant enzymes (SOD, CAT, and GSH-R) and MDA level (as the biomarker for lipid peroxidation) in the liver were shown in Table VII. A significant elevation in MDA level was observed in the rats exposed to TCDD, whereas GSH-R, SOD and CAT activities significantly declined in liver tissue in this group compared to the control group. There were also no statistically significant changes between the QCT and control groups regarding MDA, SOD, CAT, and GSH-R levels. Otherwise, the decline in MDA levels and rises in GSH-R, SOD and CAT activities in the group pre- and post-treated with QCT (QCT-TCDD and TCDD-QCT) were observed in comparison to the group exposed to TCDD. Administration of QCT thirty minutes before TCDD (pre-treatment group) significantly improved SOD, CAT, and MDA levels compared to TCDD group. However these values were lower than the control group.
P4501A1 gene expression
The results showed that although the expression of P4501A1 increased in TCDD group, was insignificant compared to control group. The statistical result of the mRNA expression level of both controls and treatment groups are shown in Figure 2.
P4501A1 mutation result
The mRNA sequence of P4501A1 gene was screened. The study was tried to find different genotypes. The codingsequence of P4501A1 gene without un-translated regions was sequenced by the geneticanalyzer (Fig. 3). The DNA sequence of P4501A1 gene was obtained from the NCBI website, to compare the resulting DNA sequences of the treatment samples (Query Sequence) with the reference sequence.
The DNA sequence of a CYP1A1 m1 gene was obtained from the NCBI website, to compare the resulting DNA sequences of patient samples (Query Sequence) with the reference sequence. The heterozygous mutation was found in the sequence of PCR template for target region (CYP1A1 m1) after comparing with the reference sequence in three samples; TCDD2, TCDD3 and TCDD-QCT2. Figure 4 indicate and reveal the sequence results.
Discussion
The of results of the study showed that body weight in the rats treated with TCDD decreaed and the liver weight increased. The results of the study is in agreement with previous findings obtained by Seefeld et al. (1984) and Ciftci et al. (2010). They showed that oral TCDD administration decreased body weight compred to control rats. This adverse effects of TCDD, including those related to the endocrine-disrupting activities (Le Magueresse-Battistoni et al., 2017; Decherf and Demeneix, 2011). Increased weight of liver can also be due to inflammation and edema due to tocixity induced bt TCDD. While, pre and post treatment with QCT could improve the liver and body weight in the rats treated with TCDD.
In fact, the increase in body weight is due to the improvement of the structure and function of the liver, which is obtained by QCT, which is consistent with the results of liver enzymes. Many studies have shown that quercetin has anti-inflammatory activity and has shown it as a potent antioxidant (Li et al., 2016; Boots et al., 2008). In the present study, significant changes in oxidative stress biomarkers and the activities of liver enzymes was observed in liver following exposure of animals to TCDD, which is in agree with previous reports (Turkez et al., 2012; Reichard et al., 2006). Various studies have indicated that TCDD through AhR-mediated signaling pathways (Turkez et al., 2012; Reichard et al., 2006) can generate various toxic effects (Turkez et al., 2012) and affect biological and biochemical responses, including oxidative damage (Reichard et al., 2006; Turkez et al., 2012; Wyde et al., 2001), cell proliferation (Reichard et al., 2006; Lucier et al., 1991), apoptosis (Reichard et al., 2006), and DNA damage (Wyde et al., 2001). It was reported that TCDD bind of the AhR (Larigot et al., 2018) and activates the dioxin response element (DRE) in the CYP1A1 gene, and induction of cytochrome P450 1A1 (CYP1A1), is a major cause of ROS-mediated oxidative damage (Kopf et al., 2010; Reichard et al., 2006; Turkez et al., 2012). Studies have shown that administration of TCDD at a dose of 1 ng/kg for 45 days causes testicular oxidative stress by inducing lipid peroxidation and hydrogen peroxide generation while suppressing antioxidant enzymes in mitochondria (Latchoumycandane et al., 2003; Wan et al., 2014). It is well known that ROS lead to oxidative damage in major hepatocytes macromolecules, such as lipids, proteins and nucleic acids, and can be caused liver and other tissues injury (Shaukat et al., 2018; Akbari et al., 2017, 2019; Heydari et al., 2016). In agreement with this evidence, the results showed that the activities of serum AST, ALT and ALP, as biochemical parameters for evaluating liver function, as well the level of MDA and the activity of SOD, GSH-R and CAT were significantly increased in TCDD-treated rats compared to the control group.
The results of the study showed that pre and post treatment of QCT to TCDD-treated groups could reverse these harmful effects. QCT was reported to improve the activity antioxidant enzymes and lipid peroxidation (Ciftci et al., 2012, 2013), and suppressed TCDD-induced toxicity in a dose-dependent manner (Ashida et al., 2000; Hamada et al., 2006). Previous studies have also shown that the used dose of QCT in this study can provide physiological levels for the responses generated (Paganga and Rice-Evans, 1997; Ashida et al., 2000; de Vries et al., 1998). In animal studies were shown that QCT could reduce the liver toxicity induced by TCDD due to its antagonistic activity against AhR (Ciftci et al., 2012, 2013). On the other hand, it has been reported that treatment of C57BL/6 mice with TCDD (15 lg/kg, i.p.) increases mitochondrial ROS, which is dependent on the AhR (Turkez et al., 2012). Other studies showed that TCDD at dose of 10 µg/kg can induce oxidative stress, DNA damage, and steatohepatitis in liver of mice (Lu et al., 2011). Shin et al. (2007) also showed that NRF2 modulates AhR signaling pathways in an exogenous ligand-independent manner. Tijet et al. (2006) identified a number of genes for which expression is AhR dependent but TCDD independent. Nebert et al. (2000) reported that the protection against ROS-induced oxidative injury has been ascribed to the AhR-mediated induction of cytoprotective genes, such as NAD (P)H: quinine oxido/reductase 1, glutathione S-transferase, and UDP-glucuronosyl transferase. While, various studies showed that QCT can improve the Nrf2 antioxidant pathway (Zaplatic et al., 2019; Rubio-Ruiz and Guarner-Lans, 2019) and possesses scavenging potential of hydroxyl radical (OH-), hydrogen peroxide (H2O2), and superoxide anion (O2-) (Zaplatic et al., 2019). In agreement with these findings, the results of the study showed that pre and post treatment of QCT can improve the level of MDA, the activity of enzymes antioxidant and liver enzymes in TCDD-treated rats.
The results of this study showed that the expression of CYP1A1 gene in the TCDD-treated group has increased, although had no significant difference with other groups, that this response is reasonable due to interactions between TCDD and AhR. This statistical difference in results may be due to the study protocol, especially the sampling time. The previous study showed that TCDD produced a sustained elevation of hepatic CYP1A2 activity, while CYP1A1 showed a transient increase, followed by a rapid loss (Reichard et al., 2006; Shertzer et al., 1998). Interestingly, it was also expected that QCT reduce the expression of CYP1A1 gene via antagonistic activity on AhR signaling pathways (Ciftci et al., 2012, 2013), while the results of the study showed that its expression increased in the QCT-TCDD and TCDD-QCT groups. Recently, there have been reports that CYP1A1 gene is expressed without the binding of any ligand to AhR (Tamaki et al., 2005; Reichard et al., 2006) by endogenous activators (Nguyen and Bradfield, 2008). Delescluse et al. (2001) showed that the expression of CYP1A1 gene may be related to the oxidants/antioxidants balance. Furthermore, other mechanisms of CYP1A1 expression, not directly explained by mediation of AhR, have been presented, namely those associated with medium change (Tamaki et al., 2005; Santes-Palacios et al., 2016).
The results of the study showed that exposure to TCDD for 90 consecutive days causes mutation in CYP1A1 gene of the liver cells. The results indicated that nucleotide sequencing for the TCDD treated sample, showing a heterozygous substitution mutation (876-T→C) in the CYP1A1 gene. In the literature, the mutagenic and genotoxic effects of TCDD are sometimes disputed (Dragan and Schrenk, 2000) and sometimes confirmed (Giri, 1986). One of the proposed mechanisms genotoxic effects of TCDD is oxidative stress and the oxygen damage to DNA (Wan et al., 2014; Gao et al., 2017). In sum, current published information related with between the mutagenic and oxidative effects of TCDD is very limited and the interpretation of these results should be done with caution. Several studies showed that the exposures of mice and rats to different doses of TCDD have resulted in increase in the production of ROS, lipid peroxidation and DNA damage (Wan et al., 2014; Gao et al., 2017; Stohs, 1990). In addition to, subchronic and chronic exposure of rats to TCDD results in dose dependent and time dependent increase in the production of ROS, lipid peroxidation and DNA damage in the whole brain tissue homogenate (Hassoun et al., 2000, 2004). In this study, we evaluated the mutagenic effects and oxidative damage of TCDD in liver cells using by measuring the activities of CAT, GSH-R and SOD enzymes and MDA level. The results showed that the level of these antioxidant enzymes increased and the level of MDA decreased after TCDD treatment and interestingly, this mutation was not observed in the TCDD treated groups receiving the quercetin. Studies have shown that the use of flavonoids such as quercetin, iron chelators and antioxidants such as catalase, superoxide dismutase and ascorbate can reduce the effect of food mutagens in human blood cells, sperm samples (Anderson et al., 1997) and Escherichia coli WP-2 uvrA and Salmonella typhimurium TA102 (Makena et al., 2009). It is likely that the mutation caused by oxidative damage or ROS-mediated intracellular signaling pathways.
The results of this study indicated that TCDD at 10µg/kg/day caused oxidative stress and mutagenic and oxidative effects in rat liver. While, pre and post treatment of quercetin (20 mg/kg/day) had strong antioxidative potentials, and it is appeared that quercetin had protective effects against the hepatotoxicity induced by TCDD. Furthermore, there is no difference between the pre and post treatment of quercetin. The beneficial effects of quercetin against TCDD-induced hepatotoxicity may be due to its antioxidant properties and its affinity for binding to the ARH receptor (Ciftci et al., 2013).
Acknowledgements
This work was supported by Salahaddin University-Erbil and approved by Ethical Research Committee with reference number 918-761-0484.
Statement of conflict of interest
There is no conflict of interest for all authors.
References
Akbari, A., Nasiri, K., Heydari, M., Mosavat, S.H. and Iraji, A., 2017. The protective effect of hydroalcoholic extract of Zingiber officinale roscoe (Ginger) on ethanol-induced reproductive toxicity in male rats. J. Evid. Based Complem.. Altern. Med., 22: 609-617. https://doi.org/10.1177/2156587216687696
Akbari, A., Nasiri, K., Heydari, M., Nimrouzi, M. and Afsar, T., 2019. Ameliorating potential of ginger (Zingiber officinale roscoe) extract on liver function and oxidative stress induced by ethanol in male rats. Zahedan J. Res. med. Sci., 21: e86464.
AL-Dhfyan, A., Alhoshani, A. and Korashy, H.M., 2017. Aryl hydrocarbon receptor/cytochrome P4501A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and Β-catenin and akt activation. Mol. Cancer, 16: 14. https://doi.org/10.1186/s12943-016-0570-y
Alharbi, N., Elobeid, M. and Virk, P., 2019. Protective effect of Quercetin treatment against cadmium-induced oxidative stress in a male rat model. Pakistan J. Zool., 51: 2287-2296 http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2287.2296
Anderson, D., Basaran, N., Dobrzynska, M.M., Basaran, A.A. and Yu, T.W., 1997. Modulating effects of flavonoids on food mutagens in human blood and sperm samples in the comet assay. Teratog. Carcinog. Mutagen., 17: 45-58. https://doi.org/10.1002/(SICI)1520-6866(1997)17:2<45::AID-TCM1>3.3.CO;2-U
Ashida, H., Fukuda, I., Yamashita, T. and Kanazawa, K., 2000. Flavones and flavonols at dietary levels inhibit a transformation of aryl hydrocarbon receptor induced by dioxin. FEBS Lett., 476: 213-217. https://doi.org/10.1016/S0014-5793(00)01730-0
Beischlag, T.V., Luis Morales, J., Hollingshead, B.D. and Perdew, G.H., 2008. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukary. Gene Expr., 18: 207-250. https://doi.org/10.1615/CritRevEukarGeneExpr.v18.i3.20
Benzie, I.F. and Strain, J.J., 1996. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem., 239: 70-76. https://doi.org/10.1006/abio.1996.0292
Boffetta, P., Mundt, K.A., Adami, H.O., Cole, P. and Mandel, J.S., 2011. TCDD and cancer: A critical review of epidemiologic studies. Crit. Rev. Toxicol., 41: 622-636. https://doi.org/10.3109/10408444.2011.560141
Boots, A.W., Wilms, L.C., Swennen, E.L., Kleinjans, J.C., Bast, A. and Haenen, G.R., 2008. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition, 24: 703-710. https://doi.org/10.1016/j.nut.2008.03.023
Brown, L.E., Trought, K.R., Bailey, C.I. and Clemons, J.H., 2005. 2, 3, 7, 8-TCDD equivalence and mutagenic activity associated with PM10 from three urban locations in New Zealand. Sci. Total Environ., 349: 61-174. https://doi.org/10.1016/j.scitotenv.2005.01.008
Bruner-Tran, K.L., Gnecco, J., Ding, T., Glore, D.R., Pensabene, V. and Osteen, K.G., 2017. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reprod. Toxicol. (Elmsford, N.Y.), 68: 59-71. https://doi.org/10.1016/j.reprotox.2016.07.007
Chang, E.T., Boffetta, P., Adami, H.O., Cole, P. and Mandel, J.S., 2014. A critical review of the epidemiology of agent orange/TCDD and prostate cancer. Eur. J. Epidemiol., 29: 667-723. https://doi.org/10.1007/s10654-014-9931-2
Ciftci, O., Aydin, M., Ozdemir, I. and Vardi, N., 2012. Quercetin prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia, 44: 164-173. https://doi.org/10.1111/j.1439-0272.2010.01126.x
Ciftci, O., Tanyildizi, S. and Godekmerdan, A., 2010. Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2,3,7,8-tetrachlorodibenzo-P-dioxin (TCDD). Immunopharm. Immunotoxicol., 32: 99-104. https://doi.org/10.3109/08923970903164318
Ciftci, O., Vardi, N. and Ozdemir, I., 2013. Effects of quercetin and chrysin on 2,3,7,8-tetrachlorodibenzo-P-dioxin induced hepatotoxicity in rats. Environ. Toxicol., 28: 146-154. https://doi.org/10.1002/tox.20707
De Pascual-Teresa, S., Johnston, K.L., Dupont, M.S., O’leary, K.A., Needs, P.W., Morgan, L.M., Clifford, M.N., Bao, Y. and Williamson, G., 2004. Quercetin metabolites downregulate cyclooxygenase-2 transcription in human lymphocytes ex vivo but not in vivo. J. Nutr., 134: 552-557. https://doi.org/10.1093/jn/134.3.552
de Vries, J.H., Hollman, P.C., Meyboom, S., Buysman, M.N., Zock, P.L., van Staveren, W.A. and Katan, M.B., 1998. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. clin. Nutr., 68: 60-65. https://doi.org/10.1093/ajcn/68.1.60
Decherf, S. and Demeneix, B.A., 2011. The obesogen hypothesis: A shift of focus from the periphery to the hypothalamus. J. Toxicol. environ. Hlth. B: Crit. Rev., 14: 423-448. https://doi.org/10.1080/10937404.2011.578561
Delescluse, C., Ledirac, N., Li, R., Piechocki, M.P., Hines, R.N., Gidrol, X. and Rahmani, R., 2001. Induction of cytochrome P450 1A1 gene expression, oxidative stress, and genotoxicity by carbaryl and thiabendazole in transfected human Hepg2 and lymphoblastoid cells. Biochem. Pharmacol., 61: 399-407. https://doi.org/10.1016/S0006-2952(00)00562-1
Dragan, Y.P. and Schrenk, D., 2000. Animal studies addressing the carcinogenicity of TCDD (or related compounds) with an emphasis on tumour promotion. Fd. Addit. Contam., 17: 289-302. https://doi.org/10.1080/026520300283360
El-Gendy, M.A.M. and El-Kadi, A.O.S., 2013. Harmine and harmaline downregulate TCDD-induced cyp1a1 in the livers and lungs of C57BL/6 mice. BioMed Res. Int.., 2013: 258095. https://doi.org/10.1155/2013/258095
Gao, L., Mutlu, E., Collins, L.B., Walker, N.J., Hartwell, H.J., Olson, J.R., Sun, W., Gold, A., Ball, L.M. and Swenberg, J.A., 2017. DNA product formation in female sprague-dawley rats following polyhalogenated aromatic hydrocarbon (PHAH) exposure. Chem. Res. Toxicol., 30: 794-803. https://doi.org/10.1021/acs.chemrestox.6b00368
Ghaffari, F. and Hajizadeh-Moghaddam, A., 2018. Neuroprotective effect of quercetin nanocrystal in A 6-Hydroxydopamine model of parkinson disease: Biochemical and behavioral evidence. Basic clin. Neurosci., 9: 317-324. https://doi.org/10.32598/bcn.9.5.317
Giri, A.K., 1986. Mutagenic and genotoxic effects of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin, A review. Mutat. Res., 168: 241-248. https://doi.org/10.1016/0165-1110(86)90022-9
Hamada, M., Satsu, H., Natsume, Y., Nishiumi, S., Fukuda, I., Ashida, H. and Shimizu, M., 2006. TCDD-induced CYP1A1 expression, an index of dioxin toxicity, is suppressed by flavonoids permeating the human intestinal Caco-2 cell monolayers. J. Agric. Fd. Chem., 54: 8891-8898. https://doi.org/10.1021/jf060944t
Hassoun, E.A., Li, F., Abushaban, A. and Stohs, S.J., 2000. The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicology, 145: 103-113. https://doi.org/10.1016/S0300-483X(99)00221-8
Hassoun, E.A., Vodhanel, J. and Abushaban, A., 2004. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J. Biochem. Mol. Toxicol., 18: 196-203. https://doi.org/10.1002/jbt.20030
Heydari, M., Heydari, H., Saadati, A., Gharehbeglou, M., Tafaroji, J. and Akbari, A., 2016. Ethnomedicine for neonatal jaundice: A cross-sectional survey in Qom, Iran. J. Ethnopharmacol., 193: 637-642. https://doi.org/10.1016/j.jep.2016.10.019
Huang, X.J., Choi, Y.K., Im, H.S., Yarimaga, O., Yoon, E. and Kim, H.S., 2006. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors, 6: 756-782. https://doi.org/10.3390/s6070756
Kopf, P.G., Scott, J.A., Agbor, L.N., Boberg, J.R., Elased, K.M., Huwe, J.K. and Walker, M.K., 2010. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced By 2,3,7,8-Tetrachlorodibenzo-P-Dioxin. Toxicol. Sci., 117: 537-546. https://doi.org/10.1093/toxsci/kfq218
Larigot, L., Juricek, L., Dairou, J. and Coumoul, X., 2018. Ahr signaling pathways and regulatory functions. Biochim. Open, 7: 1-9. https://doi.org/10.1016/j.biopen.2018.05.001
Latchoumycandane, C., Chitra, K.C. and Mathur, P.P., 2003. 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD) induces oxidative stress in the epididymis and epididymal sperm of adult rats. Arch. Toxicol., 77: 280-284. https://doi.org/10.1007/s00204-003-0439-x
Le Magueresse-Battistoni, B., Labaronne, E., Vidal, H. and Naville, D., 2017. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World J. biol. Chem., 8: 108-119. https://doi.org/10.4331/wjbc.v8.i2.108
Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M.T., Wang, S., Liu, H. and Yin, Y., 2016. Quercetin, inflammation and immunity. Nutrients, 8: 167. https://doi.org/10.3390/nu8030167
Lin, P.H., Lin, C.H., Huang, C.C., Chuang, M.C. and Lin, P., 2007. 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly (ADP-Ribose) Polymerase-1 activation in human breast carcinoma cell lines. Toxicol. Lett., 172: 146-158. https://doi.org/10.1016/j.toxlet.2007.06.003
Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J., 1951. Protein measurement with the folin phenol reagent. J. biol. Chem., 193: 265-275.
Lu, H., Cui, W. and Klaassen, C.D., 2011. Nrf2 protects against 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD)-induced oxidative injury and steatohepatitis. Toxicol. appl. Pharmacol., 256: 122-135. https://doi.org/10.1016/j.taap.2011.07.019
Lucier, G.W., Tritscher, A., Goldsworthy, T., Foley, J., Clark, G., Goldstein, J. and Maronpot, R., 1991. Ovarian hormones enhance 2,3,7,8-Tetrachlorodibenzo-P-Dioxin-mediated increases in cell proliferation and preneoplastic foci in a two-stage model for rat hepatocarcinogenesis. Cancer Res., 51: 1391-1397.
Makena, P.S., Pierce, S.C., Chung, K.T. and Sinclair, S.E., 2009. Comparative mutagenic effects of structurally similar flavonoids quercetin and taxifolin on tester strains Salmonella typhimurium TA102 and Escherichia coli WP-2 uvra. Environ. mol. Mutagen., 50: 451-459. https://doi.org/10.1002/em.20487
Nebert, D.W., Roe, A.L., Dieter, M.Z., Solis, W.A., Yang, Y. and Dalton, T.P., 2000. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem. Pharmacol., 59: 65-85. https://doi.org/10.1016/S0006-2952(99)00310-X
Ngo, A.D., Taylor, R., Roberts, C.L. and Nguyen, T.V., 2006. Association between agent orange and birth defects: Systematic review and meta-analysis. Int. J. Epidemiol., 35: 1220-1230. https://doi.org/10.1093/ije/dyl038
Nguyen, L.P. and Bradfield, C.A., 2008. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol., 21: 102-116. https://doi.org/10.1021/tx7001965
Paganga, G. and Rice-Evans, C.A., 1997. The identification of flavonoids as glycosides in human plasma. FEBS Lett., 401: 78-82. https://doi.org/10.1016/S0014-5793(96)01442-1
Patrizi, B. and Siciliani de Cumis, M., 2018. TCDD toxicity mediated by epigenetic mechanisms. Int. J. mol. Sci., 19: 4101. https://doi.org/10.3390/ijms19124101
Reichard, J.F., Dalton, T.P., Shertzer, H.G. and Puga, A., 2006. Induction of oxidative stress responses by dioxin and other ligands of the aryl hydrocarbon receptor. Dose-Response: Public. Int. Hormesis Soc., 3: 306-331. https://doi.org/10.2203/dose-response.003.03.003
Rubio-Ruiz, M.E. and Guarner-Lans, V., 2019. Resveratrol and quercetin administration improves antioxidant defenses and reduces fatty liver in metabolic syndrome rats. Molecules, 24: E1297. https://doi.org/10.3390/molecules24071297
Santes-Palacios, R., Ornelas-Ayala, D., Cabañas, N., Marroquín-Perez, A., Hernández-Magaña, A., Del Rosario Olguín-Reyes, S., Camacho-Carranza, R. and Espinosa-Aguirre, J.J., 2016. Regulation of human cytochrome P4501A1 (Hcyp1a1): A plausible target for chemoprevention? BioMed Res. Int., 2016: 5341081. https://doi.org/10.1155/2016/5341081
Seefeld, M.D., Keesey, R.E. and Peterson, R.E., 1984. Body weight regulation in rats treated with 2,3,7,8-Tetrachlorodibenzo-P-Dioxin. Toxicol. appl. Pharmacol., 76: 526-536. https://doi.org/10.1016/0041-008X(84)90357-0
Shaukat, N., Javed, M., Ambreen, F. and Latif, F., 2018. Oxidative stress biomarker in assessing the lead induced toxicity in commercially important fish, Labeo rohita. Pakistan J. Zool., 50: 735-741. http://dx.doi.org/10.17582/journal.pjz/2018.50.2.735.741
Shertzer, H.G., Nebert, D.W., Puga, A., Ary, M., Sonntag, D., Dixon, K., Robinson, L.J., Cianciolo, E. and Dalton, T.P., 1998. Dioxin causes a sustained oxidative stress response in the mouse. Biochem. biophys. Res. Commun., 253: 44-48. https://doi.org/10.1006/bbrc.1998.9753
Shin, S., Wakabayashi, N., Misra, V., Biswal, S., Lee, G.H., Agoston, E.S., Yamamoto, M. and Kensler, T.W., 2007. NRF2 modulates aryl hydrocarbon receptor signaling: Influence on adipogenesis. Mol. cell. Biol., 27: 7188-7197. https://doi.org/10.1128/MCB.00915-07
Sorg, O., Zennegg, M., Schmid, P., Fedosyuk, R., Valikhnovskyi, R., Gaide, O., Kniazevych, V. and Saurat, J.H., 2009. 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD) poisoning in victor yushchenko: Identification and measurement of TCDD metabolites. Lancet, 374: 1179-1185. https://doi.org/10.1016/S0140-6736(09)60912-0
Stohs, S.J., 1990. Oxidative stress induced by 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD). Free Radic. Biol. Med., 9: 79-90. https://doi.org/10.1016/0891-5849(90)90052-K
Tamaki, H., Sakuma, T., Uchida, Y., Jaruchotikamol, A. and Nemoto, N., 2005. Activation of CYP1A1 gene expression during primary culture of mouse hepatocytes. Toxicology, 216: 224-231. https://doi.org/10.1016/j.tox.2005.08.007
Tian, Y., Ke, S., Chen, M. and Sheng, T., 2003. Interactions between the aryl hydrocarbon receptor and P-Tefb. Sequential recruitment of transcription factors and differential phosphorylation of C-terminal domain of RNA polymerase II at Cyp1a1 promoter. J. biol. Chem., 278: 44041-44048. https://doi.org/10.1074/jbc.M306443200
Tijet, N., Boutros, P.C., Moffat, I.D., Okey, A.B., Tuomisto, J. and Pohjanvirta, R., 2006. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol. Pharmacol., 69: 140-153. https://doi.org/10.1124/mol.105.018705
Turkez, H., Geyikoglu, F., Yousef, M.I., Celik, K. and Bakir, T.O., 2012. Ameliorative effect of supplementation with L-glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-Tetrachlorodibenzo-P-Dioxin in rat hepatocyte cultures. Cytotechnology, 64: 687-699. https://doi.org/10.1007/s10616-012-9449-y
Van Den Berg, M., Birnbaum, L.S., Denison, M., de Vito, M., Farland, W., Feeley, M., Fiedler, H., Hakansson, H., Hanberg, A., Haws, L., Rose, M., Safe, S., Schrenk, D., Tohyama, C., Tritscher, A., Tuomisto, J., Tysklind, M., Walker, N. and Peterson, R.E., 2006. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci., 93: 223-241. https://doi.org/10.1093/toxsci/kfl055
Wan, C., Liu, J., Nie, X., Zhao, J., Zhou, S., Duan, Z., Tang, C., Liang, L. and Xu, G., 2014. 2, 3, 7, 8-Tetrachlorodibenzo-P-Dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS One, 9: E89811. https://doi.org/10.1371/journal.pone.0089811
Weber, R., Herold, C., Hollert, H., Kamphues, J., Blepp, M. and Ballschmiter, K., 2018. Reviewing the relevance of dioxin and PCB sources for food from animal origin and The need for their inventory, control and management. Environ. Sci. Eur., 30: 42. https://doi.org/10.1186/s12302-018-0166-9
Wiest, E.F., Walsh-Wilcox, M.T., Rothe, M., Schunck, W.H. and Walker, M.K., 2016. Dietary Omega-3 polyunsaturated fatty acids prevent vascular dysfunction and attenuate cytochrome P4501A1 expression by 2,3,7,8-Tetrachlorodibenzo-P-Dioxin. Toxicol. Sci. Off. J. Soc. Toxicol., 154: 43-54. https://doi.org/10.1093/toxsci/kfw145
Wright, P., Leathwood, P. and Plummer, D., 1972. Enzymes in rat urine: Alkaline phosphatase. Enzymologia, 42: 317-327.
Wyde, M.E., Wong, V.A., Kim, A.H., Lucier, G.W. and Walker, N.J., 2001. Induction of hepatic 8-Oxo-Deoxyguanosine adducts by 2,3,7,8-Tetrachlorodibenzo-P-Dioxin in sprague-dawley rats is female-specific and estrogen-dependent. Chem. Res. Toxicol., 14: 849-855. https://doi.org/10.1021/tx000266j
Yao, R.Q., Qi, D.S., Yu, H.L., Liu, J., Yang, L.H. and Wu, X.X., 2012. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-Trkb-PI3K/Akt signaling pathway. Neurochem. Res., 37: 2777-2786. https://doi.org/10.1007/s11064-012-0871-5
Zaplatic, E., Bule, M., Shah, S.Z.A., Uddin, M.S. and Niaz, K., 2019. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci., 224: 109-119. https://doi.org/10.1016/j.lfs.2019.03.055
To share on other social networks, click on any share button. What are these?










