Assessing Suitability of Wheat Genotypes for Growth, Yield and Nutrients Uptake under Sand Culture
Assessing Suitability of Wheat Genotypes for Growth, Yield and Nutrients Uptake under Sand Culture
Asad Ullah Khan1, Wiqar Ahmad1*, Amir Raza2, Farmanullah Khan3 and Muhammad Sharif3
1Department of Soil and Environmental Sciences, The University of Agriculture, AMK Campus Mardan, Khyber Pakhtunkhwa, Pakistan; 2Nuclear Institute for Food and Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Department of Soil and Environmental Sciences, The University of Agriculture, Main Campus Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Genetic variability among genotypes of a plant is responsible for variation in growth, yield and nutrients uptake performance. This research investigated the survival and growth efficiency of the 10 wheat (Triticum aestivum L.) genotypes under sand culture including Batoor-2007, Barsat (NRL 0320), Fakhr-e-Sharhad-99 (FS-99), Tatara-96 (WS 395), Paktunkhwa-15 (PR-103), NIFA Lalma (NRL 0517), Pirsabak 2013, NIFA Insaf (NRL 0707), Shahkar-2015 and Pirsabak 2015 (PR-105). Pots were arranged in completely randomized design in a glass-house at the Nuclear Institute for Food and Agriculture (NIFA), Peshawar, during 2016. Hoagland solution was used as irrigation and plant nutrition applied at 3 days interval. Environmental variation was minimized through constant shuffling of pots every 7th day. The genotype FS-99 showed significantly (p<0.01) higher plant height, chlorophyll content, root length, biological yield, grain N, K and Zn uptake, straw N and P accumulation compared to the rest. For root N, Pirsabak 2015 was similar to FS-99 (7.2, 7.1 mg pot-1) whilst being significantly higher over the rest. Some other genotypes were superior for specific attributes like Batoor-2007 for grain P and Straw Zn, NRL-0320 for straw K, NRL 0707 for root K and PR-103, NRL 0517 and NRL 0707 for root Zn. However, FS-99 stood distinctive upon the superior agronomic performance and nutrients accumulation attributes and is suggested for cultivation.
Received | September 05, 2017; Accepted | April 20, 2019; Published | July 05, 2019
*Correspondence | Wiqar Ahmad, Department of Soil and Environmental Sciences, The University of Agriculture, AMK Campus Mardan, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Khan, A.U., W. Ahmad, A. Raza, F. Khan and M. Sharif. 2019. Assessing suitability of wheat genotypes for growth, yield and nutrients uptake under sand culture. Sarhad Journal of Agriculture, 35(3): 741-750.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.741.750
Keywords | Grain yield, Biological yield, Nutrients accumulation, Sand culture, Wheat genotypes
Introduction
World’s population is expected to be 9 billion by 2050 (Lal, 2008; Timothy, 2013) whose feeding arrangements seem tedious because of a number of ruthless factors like water resource shortage. The only alternative left is to increase yield per unit area (Mingsheng et al., 2012) with high yielding, robust and resilient crop genotypes and technological measures.
Cereals are the top cultivated crops throughout the world, however, their growth and yield suffer a lot because of the adverse environmental signals (Shahbaz and Ashraf, 2013). Wheat (Triticum aestivum L.) is the leading grain cereal (Ashraf et al., 2013) in Pakistan facing the challenge of 1.6% per annum rise in demand (Hussain et al., 2014) either due to the rapidly growing population or changing consumption patterns. This needs wheat production to get double by 2050 (Foresight, 2011). However, in the wake of intensive climatic events, it has been predicted that there will be frequent droughts by the end of this century (IPCC, 2007) resulting in the extinction of minor crops and heavily impacted major crops. In the major wheat-growing areas, terminal drought; higher mean pan evaporation than the average precipitation especially during grain filling and lack of water availability during reproductive and grain-filling phases (Reynolds et al., 2005a) will severely affect the yield. Under situations being faced today, one important factor for wheat yield is to improve its photosynthetic rate and efficiency which require more efficient root systems (Reynolds et al., 2011). Efficient absorption of nutrients and water by roots are main contributors for the survival of plants and higher production.
Low input sustainable Agriculture (LISA) has increased awareness among scientists to search for highly production crop genotypes under low resource inputs. Genetic variability and plant’s ability to accumulate, translocate, and utilize mineral nutrient are worth in plant’s adaptability to a particular condition. These differences are responsible for the survival or failure of genotypes for large scale adoptability by the farmers. Plants with the ability to grow and produce well with low or limited amounts of mineral nutrients in soil are considered more efficient (Blume, 1988; Clark and Duncun, 1991) for low fertility soils.
Approximately 60% global soil is termed unattractive for higher production due to a variety of growth affecting factors just like mineral stress (Foy, 1983), moisture stress or soil physical properties. Plant possesses genetic differences which are expressed in terms of morpho-physiological symptoms. Such symptoms are related to growth parameters as well as the nutrient dynamics in plants like absorptions, translocations, and utilization (Gerloff, 1987; Yaseen et al. 1998). Improved wheat varieties form an integral component of production technology in Pakistan as well as in other developing countries of the region. These genotypes differ in abilities to accumulate soil nutrients and to withstand the stress.
Development and selection of wheat genotypes with satisfactory production along with site-specific set of production technology is bitterly needed to sustain crop productivity. Pakistan is among the top ten countries of the world hardly hit by climate change where advancements in the field of biotechnology, breeding and selection approaches seem indispensible to improve crop performance. Selection approach to improve growth, yield and nutrient accumulation in wheat is, therefore, indispensible to keep pace with population demand.
Understanding of the delicacies of plants efficiency for nutrient absorption is leading to evaluation of cultivars that will ultimately help in sustainability in many ways (Graham and Welch, 1996). Studies on nutrient absorption are important for the evaluation of genotypes (Akintoye et al., 1999). This is especially worth in the wake of rising extreme climatic events worldwide. Keeping in view the role of nutrients uptake in maintaining plant productivity and enhancing wheat quality, this study was conducted to identify potential wheat genotypes for improved growth and nutrient accumulation under sand culture conditions. Recommendations out of this study will, no doubt, be helpful to encourage farmers cultivating better wheat genotypes.
Materials and Methods
Treatments and experiment layout
Sand (8 kg) filled pots (height 15 inches, diameter 8 inches) were arranged according to completely randomized design (CRD) in glass-house (Figure 1) at the Nuclear Institute for Food and Agriculture (NIFA), Peshawar, Pakistan during wheat season 2015-16. Sand culture was obtained after sieving with 2 and 0.02 mm sieves and washed thoroughly with tap water before filling into the pots to remove dust and leach nutrients, if any. Ten uniform seeds of the ten selected wheat genotypes and heat tolerant accessions having wide diversity in their yield potential and genetic back ground viz Batoor-2007, Barsat (NRL 0320), Fakhr e Sharhad-99, Tatara-96 (WS 395), Paktunkhwa-15 (PR-103), NIFA Lalma (NRL 0517), Pirsabak 2013, NIFA Insaf (NRL 0707), Shahkar-2015 and Pirsabak 2015 (PR-105) were sown on 20th November, 2015 in respective pots; each one replicated three times. Pots were shuffled weekly to minimize the environmental effect if any. After germination, five uniform seedlings were maintained per pot. Hoagland and Arnon (1950) nutrient solution (Table 1) was applied at the rate of 0.5 L pot-1 at 30 days interval (Raza et al., 2016) to meet nutritional requirements of the plants. Solution pH was maintained between 6.0 and 6.5. Pots moisture content was maintained around field capacity by adding distilled water at the rate of 0.5 L pot-1 at 3 days interval throughout the study. Pots were kept open to allow leaching of excess water if any. Mean day temperature was 20 oC initially and 30 oC at latter growth stages, night temperature was 15 oC initially and 20 oC at latter growth stages. Mean photoperiod was 12 hours and relative humidity was 25%. The crop was harvested on 15th April, 2016 and data on various parameters like plant height, root length, dry weight, grain yield, biological yield, straw yield, 100 grain weight were recorded within next 5 days after crop harvest.
Table 1: Hoagland and Arnon nutrient solution.
| Reagents | Stock molarity |
Salt (g L-1) |
Stock solution for 10 L1/2 strength Hoagland solution (ml) |
Final molarity (mmol) |
| Macronutrient | ||||
|
KH2PO4 |
1 | 136 | 5 | 0.5 |
|
KNO3 |
1 | 101 | 25 | 2.5 |
|
Ca(NO3)24H2O |
1 | 236 | 25 | 2.5 |
|
MgSO4 4H2O |
1 | 246 | 10 | 1.0 |
| Micronutrients | ||||
|
H3BO3 |
2.86 | 5 | ||
|
MnCl2 4H2O |
1.81 | 5 | ||
|
ZnSO4 7H2O |
0.22 | 5 | ||
|
CuSO4 5H2O |
0.88 | 5 | ||
|
H2MoO4 H2O |
0.09 | 5 | ||
| Fe EDTA | 37.33 | 5 |
Agronomic parameters
The genotypes were compared for plant height at physiological maturity (145 days after sowing) using a meter rod, chlorophyll content at fully expanded green mid-leaf areas using SPAD-Chlorophyll meter (SPAD-502, Konica Minolta, Japan) (Raza et al., 2016). Root length was measured with a wooden rod after the crop harvest from pots, roots washed out from sand with water and spread on a paper sheet (Figure 1). Root dry weight pot-1 after Sun (Figure 1) and oven drying at temperature 700C for 24-36 hours (Pietsch et al., 2007). Grain yield (g pot-1) was recorded after threshing of spikes pot-1, biological yield after the air drying of the harvested crop pot-1 and 100 grain weight by weighing 100 grains from each treatment with the help of sensitive balance.
Laboratory analysis
Soil as well as plant total N was estimated by the Kjeldahl method (Bremner and Mulvaney, 1982). A 0.2 g of sample was digested with H2SO4 (3 mL) and K2SO4, CuSO4 and Se (digestion mixture ) on block digester for 4-5 hours starting from 50 °C to 100, 150, 200, 300 and 350 °C and maintained the final temperature for 1 hour (till the sample turned light greenish or colorless). The sample was then cooled and diluted to 100 mL with distilled water. A 20 mL of the diluted sample was distilled in Boric acid mixed indicator in the presence of 5 mL 40% NaOH solution and titrated against standard 0.005 m HC1. A blank was also run concurrently and subtracted its reading from the sample. N-uptake in grain, straw and root were calculated as follows.
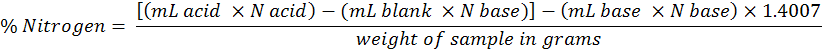
Where;
1.4007 = milli equivalent weight of nitrogen × 100
The nutrients uptake in crop was = root/straw/grain dry weight (g pot-1)*respective nutrient content (g pot-1).
Phosphorus total was estimated by method outlined in (AOAC, 1979). A 2 g sample was mixed with 10 mL of perchloric and nitric acid mixture (digestion mixture) and placed on hot plate and increased the temperature slowly from 100 to 200 oC until the appearance of white fumes. A few ml of distilled water was added and left to cool. The sample was then diluted to 50 ml. A 5 ml of the sample was taken from it in a 50 ml volumetric flask, added 10 ml of color developing ammonium vanadomolybdate solution reagent and made volume up to 50 ml with distilled water. The reading was read with spectronic 20 at 880 nm. The sample digested for phosphorus was read for potassium using flame photometer and for Zinc concentration using atomic absorption spectrophotometer (Perrkin Elmer 2380) using respective element cathode lamp.
Statistical analysis
The data obtained were analyzed statistically by computer using STATX 8.0 through analysis of variance (ANOVA) procedure for completely randomized design (CRD). Significantly different means were compared through Least Significant Difference (LSD) test (Steel and Torrie, 1980).
Results and Discussion
In the current experiment, ten wheat genotypes grown under sand culture conditions indicated significant (P≤ 0.05) variation in plant height, chlorophyll content, root length and dry root biomass amongst the genotypes (Table 2). Fakhar-e-Sarhad-99 (FS-99) exhibited significantly increased plant height (79.33 cm), chlorophyll content (47 %), root length (45.3 cm) and dry root biomass (2.47 g plant-1) compared to the rest. Plant height and chlorophyll content of the recently released genotypes Pirsabak-2013 and Shahkar-2015 was lower by 43 and 18.5%, respectively, than the FS-99. Root length showed highly significant correlation with plant height and chlorophyll content (Figure 2, r2 = 0.86 and 0.79 respectively). The lower root length of Pakhtunkhwa-15 (PR 103) as well as Tatara-96 (WS 395) by 21 and 24% with corresponding lower plant height and chlorophyll content over FS-99, respectively, also supported this statement. Furthermore, dry root biomass in Shahkar-2015 was 70% lower than FS-99. Amongst the currently in use wheat genotypes, Pirsabak-2015 and Barsat (NRL-0320) performance was better next to FS-99 (3 and 3.4% difference, respectively) whilst the NIFA Insaf (NRL 0707) and Batoor 2007 were intermediate in plant height (16 and 16.6% difference FS-99, respectively). Root length amongst the genotypes was the highest for FS-99 followed by NRL 0320 whilst it was lowest for NRL 0517.
Wheat genotypes significantly (P≤ 0.05) differed in grain yield (Table 3) with NRL 0320, Batoor-2007 and PR-105 as the better performers whilst all the three being statistically similar. The FS-99 showed 11% lower grain yield than NRL-0320 and ranked second in grain yield, however, it stood statistically at par with Batoor and PR-105 (with 9 and 3% differences in grain yield) and was therefore, successful in competition under sand culture conditions. Grain yield by NRL-0517 was the lowest amongst the genotypes. On the other hand genotype NRL-0320 ranked only second in root length, root dry weight and P uptake to the FS-99 that helped to attain the highest grain yield.
Table 2: Plant height (cm), chlorophyll content, root length (cm) and root dry weight plant-1 as affected by wheat genotypes.
| Plant | Chlorophyll | Rooting | Root dry | |
| Wheat Genotypes | Height | Content | Depth | Weight |
| (cm) | (%) | (cm) | plant-1 (g) | |
| Batoor-2007 | 68.00 c | 43.33 bc | 39.33 de | 2.04 ab |
| Barsat (NRL 0320) | 76.67 b | 44.67 ab | 42.33 b | 1.89 bc |
| Fakhar-e-Sarhad-99 | 79.33 a | 47.00 a | 45.33 a | 2.47 a |
| Tatara (WS 395)-96 | 58.00 e | 40.00 d | 37.67 ef | 1.54 bc |
| Pakhtunkhwa-15 (PR 103) | 60.67 d | 41.33 cd | 36.67 f | 1.60 bc |
| NIFA Lalma (NRL 0517) | 55.67 f | 39.00 d | 33.67 g | 1.37 c |
| Pirsabak-2013 | 55.33 f | 39.67 d | 38.67 de | 1.86 bc |
| NIFA Insaf (NRL 0707) | 68.33 c | 43.00 bc | 40.00 cd | 2.10 ab |
| Shahkar-2015 | 58.00 e | 39.67 d | 36.33 f | 1.45 c |
| Pirsabak-2015 (PR 105) | 77.00 b | 44.00 b | 41.33 bc | 2.46 a |
| LSD value (p<0.05) | 1.319 | 2.564 | 1.865 | 0.558 |
Table 3: Grain yield (g), 100 grain weight (g), and Biological yield (g) as affected by wheat genotypes.
| heat Genotypes |
Grain Yield (g pot-1) |
100 grain Weight (g) |
Biological Yield (g pot-1) |
| Batoor-2007 | 3.97 ab | 3.19 a | 12.17 b |
| Barsat (NRL 0320) | 4.03 a | 1.82 de | 13.30 ab |
| Fakhar-e-Sarhad-99 (FS-99) | 3.63 bc | 1.35 f | 14.77 a |
| Tatara (WS 395)-96 | 2.93 e | 1.52 ef | 12.17 b |
| Pakhtunkhwa-15 (PR-103) | 3.00 de | 2.98 ab | 11.83 bc |
| NIFA Lalma (NRL 0517) | 2.70 e | 2.64 bc | 13.20 b |
| Pirsabak 2013 | 2.73 e | 2.00 d | 9.53 d |
| NIFA Insaf (NRL 0707) | 3.37 cd | 2.89 ab | 12.10 b |
| Shahkar-2015 | 2.93 e | 2.21 cd | 9.53 d |
| Pirsabak 2015 (PR-105) | 3.73 abc | 2.68 b | 10.33 cd |
| LSD value (p<0.05) | 0.367 | 0.446 | 1.547 |
Wheat genotypes Batoor-2007, PR-103 and NRL 0707 ranked first in 100 grain weight (3.2, 3 and 2.9 g, respectively) all being statistically similar whilst the FS-99 and WS-395 were minimum (1.4 and 1.5 g, respectively) both being statistically at par. FS-99 and NRL 0320 ranked the leaders in biological yield both being statistically similar (Table 3) whilst the newer genotypes Pirsabak-2013 and Shahkar-2015 were the lowest (by 40% over FS-99). The FS-99 was significantly (p<0.05) higher in grain N, K and Zn whilst fourth but statistically similar to the maximum (PR-103) in grain P (Table 4). Close followers of the FS-99 in grain N were NRL-0320 and NRL 0707, respectively, whereas, Pirsabak-2013 and Shahkar-2015 fell at the end of the list whilst all others were intermediate. The order and their statistical similarity of the top five genotypes in grain P content was PR-103 > NRL 0517 > Batoor 2007 > WS 395 > FS-99 > Pirsabak 2013 kept all of them distinguished amongst the list whilst in the grain K, the order of top 7 but statistically similar genotypes (FS-99 > WS 395 > PR-103 > NRL-0320 > NRL 0517 > Batoor 2007) kept FS-99 on the top. In grain Zn content, FS-99 was the sole significantly (p<0.05) higher genotype over the rest. Grain Zn content showed nearly significant positive correlation with root length and grain N content (Figure 2, r2 = 0.44 and 0.39, respectively) might support these results. Results (Table 4) further indicated that Shahkar 2015 and Pirsabak-2013 were both the lowest in grain N and K content, Shahkar 2015 in the grain P and Pirsabak 2013 in the grain Zn content. Genotype PR-103 was amongst the significantly higher (p<0.05) accumulator of grain P and K but was significantly (p<0.05) poor in grain N and Zn uptake over the FS-99, genotype PR 105 was statistically at par with FS-99 in grain P accumulation but was significantly lower to it in grain N, K and Zn. Rest of the genotypes proved intermediate accumulators of grain N, P, K and Zn.
Table 4: Grain N, P, K and Zn content (mg pot-1) as affected by wheat genotypes.
| Wheat Genotypes | Grains N | Grains P | Grains K | Grains Zn |
| (mg pot-1) | (µg pot-1) | |||
| Batoor-2007 | 6.49 bc | 3.14 a | 4.87 abc | 2.00 de |
| Barsat (NRL 0320) | 8.51 a | 2.37 d | 5.32 a | 1.82 def |
| Fakhar-e-Sarhad-99 | 8.86 a | 3.00 ab | 5.91 a | 6.04 a |
| Tatara-96 (WS 395) | 6.81 b | 3.02 ab | 5.69 a | 2.38 cd |
| Pakhtunkhwa-15 (PR-103) | 6.47 bc | 3.21 a | 5.33 a | 1.32 f |
| NIFA Lalma (NRL 0517) | 6.86 b | 3.19 a | 5.28 ab | 2.13 cd |
| Pirsabak-2013 | 4.71 d | 2.89 abc | 3.81 c | 1.48 ef |
| NIFA Insaf (NRL 0707) | 7.08 b | 2.57 bcd | 4.84 abc | 3.41 b |
| Shahkar-2015 | 5.50 cd | 2.46 cd | 3.81 c | 2.15 cd |
| Pirsabak-2015 (PR-105) | 6.48 bc | 2.76abcd | 4.13 bc | 2.63 c |
| LSD value (p<0.05) | 1.026 | 0.460 | 1.169 | 0.607 |
Genotypes also exhibited significantly (P≤ 0.05) different straw N, P, K and Zn content (Table 5). Results indicated the FS-99 as the significantly higher accumulator for straw N closely followed by NRL 0517 both being statistically similar. In straw P, FS-99 was the sole significantly higher accumulator whilst in straw K, the order of the top and significantly higher accumulator genotypes was NRL 0320 > FS-99 > PR-103 > WS 395 > Batoor-2007 > NRL 0707 > NRL 0517 all being statistically similar. In straw Zn content, genotype Batoor-2007 was the sole significantly high accumulator followed by NRL 0320, FS-99 and NRL 0707 all the three being statistically similar. Results further indicated that Pirsabak-2013 and PR-105 were the lowest in straw N, Pirsabak-2013 and Shahkar-2015 were the lowest in straw P and K and Pirsabak-2013, Shahkar-2015, PR-105 and NRL 0517 were the lowest in straw Zn whilst rest of the genotypes were intermediate in straw N, P, K and Zn.
Wheat genotypes were significantly (P≤ 0.05) different in the root N, P, K and Zn content and accumulation (Table 6). PR-105 was the highest accumulator for root N and was statistically similar to FS-99. The significantly lowest root N accumulator genotypes were Pirsabak-2013 and NRL 0517. The maximum root P content was recorded in NRL 0517, closely followed by and statistically similar with FS-99 and PR-103. The minimum and significantly lowest root P content was reported for Shahkar-2015, Pirsabak-2013 and PR-105, all the three being statistically at par. Only PR-105 was statistically at par to FS-99 in grain P whilst all these were lower in straw P compared to FS-99. Wheat genotype NRL 0707 resulted in higher root K whereas FS-99 revealed to be the lower intermediate in root K and being statistically at par with Batoor-2007, NRL 0320 and PR-105. The highest root Zn content was noted with NRL 0707, PR-103 and NRL 0517 (Table 6) all being statistically similar. Batoor-2007 and NRL 0320 showed the lowest root Zn content whilst rest of the genotypes including FS-99 and Shahkar-2015 were intermediate in root Zn content and accumulation.
Table 5: Straw N, P, K and Zn content (µg pot-1) as affected by Wheat genotypes.
| Wheat Genotypes | Straw N | Straw P | Straw K | Straw Zn |
|
(mg pot-1) |
(µg pot-1) |
|||
| Batoor-2007 | 2.11 bcd | 0.77 b | 24.73 ab | 4.82 a |
| Barsat (NRL 0320) | 2.49 bcd | 0.76 b | 25.71 a | 2.47 b |
| Fakhar-e-Sarhad-99 | 3.34 a | 0.95 a | 25.58 ab | 2.41 b |
| Tatara-96 (WS 395) | 2.44 bcd | 0.63 c | 25.15 ab | 1.79 cd |
| Pakhtunkhwa-15 (PR-103) | 2.21 bcd | 0.66 bc | 25.44 ab | 1.61 cd |
| NIFA Lalma (NRL 0517) | 2.83 ab | 0.72 bc | 24.22 ab | 1.54 d |
| Pirsabak-2013 | 1.79 d | 0.42 d | 20.05 c | 1.29 d |
| NIFA Insaf (NRL 0707) | 2.56 bc | 0.69 bc | 24.60 ab | 2.12 bc |
| Shahkar-2015 | 2.18 bcd | 0.42 d | 20.58 c | 1.26 d |
| Pirsabak-2015 (PR-105) | 2.05 cd | 0.63 c | 22.40 bc | 1.46 d |
| LSD value (p<0.05) | 0.737 | 0.120 | 3.180 | 0.535 |
Table 6: Root N, P, K and Zn content (µg pot-1) as affected by wheat genotypes.
| Wheat Genotypes | Root N | Root P | Root K | Root Zn |
|
(mg pot-1) |
(µg pot-1) |
|||
| Batoor-2007 | 5.07 d | 0.61 bc | 2.05 de | 1.43 e |
| Barsat (NRL 0320) | 6.63 b | 0.60 bc | 2.35 cde | 2.71 de |
| Fakhar-e-Sarhad-99 | 7.10 a | 0.77 a | 1.92 e | 4.76 c |
| Tatara-96 (WS 395) | 4.27 e | 0.61 bc | 2.59 bcd | 3.29 d |
| Pakhtunkhwa-15 (PR-103) | 5.43 c | 0.68 ab | 2.93 bc | 8.21 a |
| NIFA Lalma (NRL 0517) | 3.33 f | 0.79 a | 2.85 bc | 8.61 a |
| Pirsabak-2013 | 3.13 f | 0.52 cd | 3.13 b | 6.43 b |
| NIFA Insaf (NRL 0707) | 6.83 b | 0.64 bc | 3.84 a | 9.10 a |
| Shahkar-2015 | 4.23 e | 0.48 d | 3.01 bc | 4.82 c |
| Pirsabak-2015 (PR-105) | 7.17 a | 0.54 cd | 2.09 de | 5.75 bc |
| LSD value (p<0.05) | 0.201 | 0.115 | 0.666 | 1.462 |
Wheat genotypes producing more biomass and yield are needed for sustainable crop productivity. Under sand culture, significantly higher plant height, chlorophyll content, root length and dry root biomass by genotype Fakhar-e-Sarhad-99 (FS-99) indicated its potential adoptability and performance. Its maximum root length (Table 2) might be helpful in scavenging the nutrients and moisture from greater soil volume resulting in maximum height, chlorophyll and biomass. Data was clearly indicative of this root-shoot and root-chlorophyll significant correlation (r2 = 0.86 and 0.79, respectively; Figure 2). The lower plant height and chlorophyll content of the Pirsabak-2013 and Shahkar-2015 (by 43 and 18.5%, respectively) and lower root biomass by Shahkar-2015 (by 70%) than the FS-99 might be ascribed to their significantly lower root length over the FS-99. Previous work (Raza et al., 2014) indicated the FS-99 as being the longer rooted and most responsive to the applied N under field conditions, showed higher nutrients uptake and a higher yield. This support our results for FS-99 with maximum root length, N uptake and chlorophyll (r2 value 0.57; Figure 2). Fallovo et al. (2009) described that increasing chlorophyll, macronutrients content and yield was likely the result of increasing availability of nutrient in soil solution and no doubt its absorption requires a deeper and vigorous rooting system. Genotypic and crop species differences exist (Bakhsh et al., 2012) resulting in significantly variable response to factors for growth and yield (Saleem et al., 2013). The root dry weight differences among the wheat genotypes might be ascribed to differences in their abilities to absorb nutrient and water and convert them into biomass. Genotypes with better nutrient absorption result in the higher rate of dry matter production subject to the required amount of light availability and the other factors (Saleem et al., 2011).
The FS-99 ranked second to NRL-0320 (with 11% difference) in grain yield and stood statistically at par with Batoor and PR-105 (with 9 and 3% difference, respectively) (Table 3). Looking into the data for rest of the growth and yield variable e.g. chlorophyll content (Table 2), grain N and K (Table 4) and straw K (Table 5) contents, the genotype NRL-0320 was the close competitor with FS-99 and all these attributes helped NRL-0320 to attain the highest grain yield (Table 3). Results are in agreement with Naidoo (2009) showing increase in plant height, chlorophyll, rate of photosynthesis and nutrient use efficiencies with increase in nutrient availability. Naidoo (2009) and Lovelock et al. (2009) ascribed this to the shift of resource allocation to shoots which increased the growth and productivity with nutrient enrichment of plants. Efficient cultivars could efficiently take up nutrients (Epstein and Bloom, 2005) and higher grain yield and stable harvest index are the main characteristics of such cultivars because of improved dry matter partitioning to the grains (Echarte et al., 2000). This is why geneticists and breeders may target more nutrient use efficient genotypes (Bakhsh et al., 2010).
Higher 100 grain weight supports total grain yield as indicated in case of NRL-320 and Batoor-2007 genotypes (Table 3). The lowest 100 grain weight might have dropped the FS-99 to the second position in grain yield (Table 3), however, its position as a second competitor in grain yield might be due its higher number of fertile spikelet. Maqsood et al. (2014) ascribed the higher 100 grain weight to more photosynthetic active radiations and supplementary N uptake promoting vegetative and reproductive growth. The distinction of FS-99 with maximum growth (Table 2) and N content (Table 5) are the result of nutrients absorption for the formation of photo-assimilates. Data on grain and straw N, P, K and Zn content (Tables 4 and 5) confirmed the leading role of FS-99 in growth and yield parameters. The maximum root length and dry root biomass by the FS-99 (Table 2) might be mainly responsible for higher absorption and translocation of nutrients into the above ground parts of the plant. Both higher root length as well as higher grain N content in the FS-99 showing positive correlation (r2 = 0.44 and 0.39, respectively, Figure 2) with grain Zn content might support these results. Previous worker reported synergistic effect in N, P, K uptake and Zn application (El-Azab, 2015) as well as increased yield for FS-99 as we; as 77% more Zn accumulator and significantly higher N, P and K up-taker among genotypes indicating that transfer of the absorbed nutrients from roots and the photoassimilates from aerial parts to grains was not the same. Such differences are mainly due to genotypic and crop species variation (Bakhsh et al., 2012) affecting the grain filling duration (Echarte et al., 2000), plant growth rate (Echarte et al., 2000; Maddonni and Otegui, 2004), sink capacity (Borras and Westgate, 2006; Gambin et al., 2006) and yield (Saleem et al., 2013).
Phosphorus is most important for seed development (Seng et al., 1994). Under optimal conditions, adequate P level in plants is about 4.0 mg g-1 dry weights (Reuter and Robinson, 1997). In our study, the higher P uptake by FS-99 is indicative of its absorption efficiency and can be classified as P efficient genotype. FS-99 was the second most efficient straw K and Zn accumulator (Table 5). Like grain quality, FS-99 was also superior in its straw quality in terms of N, P, K and was only second after Batoor-2007 in straw Zn (Table 5). Being inferior in other parameters, Batoor-2007 cannot be recommended. However, under specific circumstances, competitor genotypes with FS-99 like NRL-0320 and NRL 0517 should be prioritized being superior in grain and straw nutritional qualities after FS-99.. Large genotypic variation exists in the same crop (Houmanat et al., 2016) resulting in different nutrient absorption and translocation to aerial parts and grain (Guopang et al., 1999). Different genotypes uptake and accumulate Zn differently (Khoshgoftarmanesh et al., 2009) from the soil or applied Zn fertilizers and Zn efficient genotypes mostly yield higher than others. In our results, FS-99 seem efficient Zn absorbers and translocators into grain so can be termed as the most desirable. Shahkar, 2015 was lowest in grain N, P, K and Pirsabak-2013 in grain N, K and Zn content (Table 4). Genotype PR-103 higher in grain P and K but poor in grain N and Zn, PR 105 was higher in grain Zn but poor in grain N, P and K and all, therefore, lost their edge. Rest of the genotypes proved intermediate accumulators of grain (Table 4) and straw N, P, K and Zn (Table 5) especially Batoor-2007, NRL 0517 and NRL 0707 and are worth considering for cultivation after the FS-99.
Both PR-105 and FS-99 were the highest accumulators for root N (Table 6) but PR-105 was significantly lower in grain and straw N content compared to FS-99 (Tables 4 and 5). The higher root, straw and grain N content in FS-99 (Tables 4, 5, 6) are testimonial of its efficient N absorption and translocation towards shoots and grains. The significantly lowest in root N, were Pirsabak-2013 and NRL 0517 (Table 6), Pirsabak-2013 seem to be lower N absorber since it was also lower in shoot and grain N whilst NRL 0517, being intermediate in grain and higher in straw N seem efficient translocator. Raza et al. (2014) reported FS-99 as best for root N due to longer root length. The root P content was maximum in NRL 0517 closely followed by statistically similar root P values of FS-99 and PR-103. NRL 0517, being inferior to FS-99 in straw P proved to be the next efficient absorber and translocator of P towards shoot and grain after FS-99. Higher N and P content in root, shoot and grain of FS-99 is indicative of synergism between both elements. Similar findings were also noted by Brink et al. (2001) reporting that appropriate proportion of nutrients in the medium facilitate their uptake by plants. FS-99 was the lower intermediate in root K. However, its higher grain and shoot K content is testimonial of its higher absorption but early translocation towards the aerial parts. K+ exhibits long distance cycle between roots and shoots due to its highly mobile nature within the plants (Szczerba et al., 2009). Based on the N, P, K and Zn accumulation, the FS-99 was on the top in N and P and intermediate in K and Zn accumulation whilst NRL 0517 was the leading genotype in Zn and P, intermediate in K but the weakest in N accumulation and thus conclusively fall inferior to FS-99. However, the NRL 0707 being the leading accumulator in Zn and K and intermediate in P and second in N after FS-99 can be considered as the second choice. Higher root N and P in FS-99 indicated its efficiency to take up these nutrients with the help of its deepest roots and maximum root biomass. Higher content of these nutrients in roots is responsible for their higher translocation towards the shoot and grain to improve the grain and straw yield and quality of the cropunder the condition of the experiment
Conclusions and Recommendations
Significantly higher nutrients accumulation by Fakhar-e-Sarhad-99 (FS-99), maximum root length and root dry weight and maximum K and Zn content in grain and straw are indicative of its efficiency for yield and quality. The NIFA Insaf (NRL 0707), being the leading in Zn and K, intermediate in P and second in N accumulation, could be considered as the second choice. In the absence of FS-99 and NIFA Insaf (NRL 0707), genotypes Barsat (NRL-0320) and NIFA Lalama (NRL 0517) should be prioritized being superior in grain nutritional qualities compered to rest of the competitors.
Acknowledgements
Authors are highly thankful to Nuclear Institute for Food and Agriculture (NIFA), Peshawar for providing all facilities for the experiment and laboratory analysis.
Novelty Statement
This study identify potential wheat genotypes for improved growth and nutrient accumulation and the recommendations will be helpful to farmers for cultivating better wheat genotypes.
Author’s Contribution
Asad Ullah Khan: Conducted the research.
Wiqar Ahmad: Supervised the research work, carried out statistical analysis.
Amir Raza: Supervised the research, helped in experiment setup.
Farmanullah Khan: Helped in laboratory, data entry and data analysis.
Muhammad Sharif: Helped in manuscript write up.
References
Al-Azab, M.E. 2015. Increasing Zn ratio in a compound foliar NPK fertilizer in relation to growth, yield and quality of corn plant. J. Innov. Pharm. Biol. Sci. 2(4): 451-468.
Akintoye, H.A., J.G. Kling and E.O. Lucas. 1999. N use efficiency of single, double and energetic maize lines grown at four N levels in three ecological zones of West Africa. Field Crops Res. 60: 189-199. https://doi.org/10.1016/S0378-4290(98)00122-1
AOAC. 1979. Official methods of analysis of the association of official analytical chemists. Washington, DC. 2044.
Ashraf, M.Y., N. Rafiq, M. Ashraf, N. Azhar and M. Marchand. 2013. Effect of supplemental potassium (K+) on growth, physiological and biochemical attributes of wheat grown under saline conditions. J. Plant Nutr. 36(3): 443-458. https://doi.org/10.1080/01904167.2012.748065
Ben-Miflin. 2000. Crop improvement in the 21st century. J. Exp. Bot. 51: 1–8. https://doi.org/10.1093/jxb/51.342.1
Blume, A. 1988. Plant breeding for stress environment. CRC Press, Inc. Boca Raton, Florida USA.
Borras, L. and M.E. Westgate. 2006. Predicting maize kernel sink capacity early in development. Field Crops Res. 95: 223-233. https://doi.org/10.1016/j.fcr.2005.03.001
Bakhsh, M.A.A.H.A., R. Ahmad, J. Iqbal. M.M. Maqbool, A. Ali, M. Ishaque and S. Hussain. 2012. Nutritional and physiological significance of potassium application in maize hybrid crop production. Pak. J. Nutr. 11 (2): 187-202. https://doi.org/10.3923/pjn.2012.187.202
Bakhsh, M.A.A.H.A., R. Ahmad, A.U. Malik, S. Hussain and M. Ishaque. 2010. Agro-physiological traits of three maize hybrids as influenced by varying potassium application. Life Sci. Int. J. 4: 1487-1496.
Bremner, J.M. and C.S. Mulvaney. 1982. Nitrogen total P.595-624. In: Methods of soil analysis part-2. 2nd edition. A.L. page, R.H. Miller and D.R. Keeney (eds). Am. Soc. Agron. Madison, W.I. USA.
Brink, G.E., G.A. Peterson, K.R. Sistani and T.E. Fairbrother. 2001. Uptake of selected nutrients by temperate grasses and legumes. Agron. J. 93: 887-890. https://doi.org/10.2134/agronj2001.934887x
Clark, R.B. and R.R. Duncan. 1991. improvement of plant mineral nutrition through breeding. Field Crop Res., 27: 219-246. https://doi.org/10.1016/0378-4290(91)90063-2
Echarte, L., S. Luque, F.H. Andrade, V.O. Sadras, A. Cirilo, M.E. Otegui and C.R.C. Vega, 2000. Response of maize kernel number to plant density in Argentinean hybrids released between 1965 and 1993. Field Crop Res. 68: 1-8. https://doi.org/10.1016/S0378-4290(00)00101-5
Epstein, E. and A. Bloom, 2005. Mineral nutrition of plants: principles and perspectives, 2. Sinauer associates, Sunderland. MA.
Fallovo, C., Y. Rouphael, E. Rea, A. Battistelli and G. Colla. 2009. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 89: 1682–1689. https://doi.org/10.1002/jsfa.3641
Farooq, M., M. Hussain and K.H.M. Siddique. 2014. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 33(4):331-349. https://doi.org/10.1080/07352689.2014.875291
Foresight. 2011. The future of food and farming: challenges and choices for global sustainability. Final project report. Govt. Off. Sci. London.
Foy, C.D. 1983. Plant adaptation to mineral stress in problem soils. Lowa St. J. Res. 57: 339–354.
Gambin, B.L., L. Borras and M.E. Otegui. 2006. Source-sink relations and kernel weight differences in maize temperate hybrids. Field Crop Res. 95: 316-326. https://doi.org/10.1016/j.fcr.2005.04.002
Gerloff, G.C. 1987. Intact plant screening for tolerance of nutrient deficiency stress. Plant Soil. 99: 3-16. https://doi.org/10.1007/BF02370149
Graham, R.D. and R.M. Welch. 1996. Breeding for staple-food crops with high micronutrient density. In: International workshop on food policy and agricultural technology to improve diet quality and nutrition. Agricultural strategies for micronutrients, working paper No. 3. Washington, D.C. Int. Food Policy Res. Inst. pp. 82.
Guopang, Z., C. Jingxing and E.A. Tirore. 1999. Genotypic variation for potassium uptake and utilization efficiency in wheat. Nut. Cycle. Agroecosyst. 59: 41-48. https://doi.org/10.1023/A:1009708012381
Hoagland, D.R. and D.I. Arnon. 1950. The water culture methods for growing plants without soil. Berkeley calif: College of agriculture, Univ. Calif., Davis Libr. Calif. Agric. Exp. Stat. Circ. C347 pp:1-32.
Houmanat, K., J. Charafi, H. Mazouz, M. El Fechtali and A. Nabloussi. 2016. Genetic diversity analysis of safflower (Carthamus tinctorius) accessions from different geographic origins using ISSR markers. Int. J. Agric. Biol. 18(6): 1081-1087. https://doi.org/10.17957/IJAB/15.0144
Hussain, A., Z.U. Zafar and H.R. Athar. 2014. Flooding tolerance in cotton G. hirsutum L. at early vegetative and reproductive growth stages. Pak. J. Bot. 46 (3):1001-1009.
IPCC. 2007. Fourth assessment report: synthesis. Published online 17 November 2007, www.ipcc.ch/ipccreports/ar4-syr.htm
Khoshgoftarmanesh, A.H., M. Zare, M. Norouzi and R. Schulin. 2009. Critical soil zinc deficiency concentration and tissue Fe:Zn ratio as diagnostic tool for prediction of zinc deficiency in corn. J. Plant Nutr. 32(12): 1983-1993. https://doi.org/10.1080/01904160903308101
Lal, R. 2008. Soils and sustainable agriculture; A review. Agron. Sust. Dev. 28(1): 57-64. https://doi.org/10.1051/agro:2007025
Lovelock, C.E., M.C. Ball, K.C. Martin and I.C. Feller. 2009. Nutrient enrichment increases mortality of mangroves. PLos One. 4 (5): e5600. https://doi.org/10.1371/journal.pone.0005600
Maddonni, G.A. and M.E. Otegui. 2004. Intra-specific competition in maize: early establishment of hierarchies among plants affects final kernel set. Field Crops Res. 85: 1-13. https://doi.org/10.1016/S0378-4290(03)00104-7
Maqsood, M., M.A. Shehzad, Y. Ramzan and A. Sattar. 2014. Effect of nitrogen nutrition on growth, yield and radiation use efficiency of different wheat (Triticum aestivum L.) cultivars. Pak. J. Agri. Sci. 51: 451-458.
Mingsheng, F., J. Shen, L. Yuan, R. Jiang, X. Chen, W.J. Davies and F. Zhang. 2012. Improving crop productivity and resource use efficiency to ensure food security and environmental quality in China. J. Exp. Bot. 63(1): 13–24. https://doi.org/10.1093/jxb/err248
Naidoo, G. 2009. Differential effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves. Aqu. Bot. 90 (2): 184-190. https://doi.org/10.1016/j.aquabot.2008.10.001
Pietsch, G., J.K. Friedel and B. Freyer. 2007. Lucerne management in an organic farming system under dry site condition. Field Crops Res. 102 (2): 104-118. https://doi.org/10.1016/j.fcr.2007.03.003
Raza, A., M. Imtiaz and W. Mohammad. 2014. Root research for sustainable use of natural resources. J. Sci. Res. Rev. 3(1):001-007.
Raza, A., M. Imtiaz and W. Mohammad. 2016. Identification of nitrogen-use-efficient and deep rooted wheat genotypes. Pak. J. Agric. Agric. Eng. Vet. Sci. 32 (1): 1-8.
Reuter, D.J. and J.B. Robinson. 1997. Plant analysis: An interpretation manual, 2nd edn., CSIRO Publishing, Australia. https://doi.org/10.1071/9780643101265
Reynolds, M.P., A. Mujeeb-Kazi and M. Sawkins. 2005a. Prospects for utilising plant-adaptive mechanisms to improve wheat and other crops in droughtand salinity-prone environments. Ann. Appl. Biol. 146: 239–259. https://doi.org/10.1111/j.1744-7348.2005.040058.x
Reynolds, M., D. Bonnett and S.C. Chapman. 2011. Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J. Exp. Bot. 62 (2): 439-452. https://doi.org/10.1093/jxb/erq311
Saleem, M.Y., K.P. Akhtar, M. Asghar, Q. Iqbal and A. Rehman. 2011. Genetic control of late blight, yield and some yield related traits in tomato (Solanum lycopersicum L.). Pak. J. Bot. 43(5): 2601-2605.
Saleem, M.Y., M. Asghar, Q. Iqbal, A.U. Rahman and M. Akram. 2013. Diallel analysis of yield and some yield components in tomato (Solanum lycopersicuml). Pak. J. Bot. 45(4): 1247-1250.
Seng, V., R.W. Bell, I.R. Willet and H.J. Nesbitt. 1994. Phosphorus nutrition of rice relation to floating and temporary loss of soil water saturation in lowland soils of Cambodia. Plant Soil. 207: 121-132.
Shahbaz, M. and M. Ashraf. 2013. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 32: 237-249. https://doi.org/10.1080/07352689.2013.758544
Sinclair, T. R., L.C. Purcell and C.H. Snelle. 2004. Crop transformation and the challenge to increase yield potential. Trends in Plant Sci. 9: 70–75. https://doi.org/10.1016/j.tplants.2003.12.008
Steel, R.G.D and J.H. Torrie. 1980. Principles and procedure of statistics: A biometrical approach. McGraw-Hill, N.Y. 2nd edition. Pp.633.
Szczerba, M.W., D.T. Britto and H.J. Kronzucker. 2009. K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 166: 447-466. https://doi.org/10.1016/j.jplph.2008.12.009
Timothy, A.W. 2013. Can we feed the world in 2050? A scoping paper to assess the evidence. Global development and environment institute working paper no.13-04. Tufts Univ. Medford M.A. 02155, USA. http://ase.tufts.edu/gdae
Yaseen, M., M.A. Gill, M. Siddique, Z. Ahmad, T. Mahood and H.U. Rehman. 1998. Phosphorus deficiency stress tolerance and phosphorus utilization efficiency in wheat genotypes. Procd. Symp. Plant Nutr. Manage. Sust. Agric. Growth, NFDC. Islamabd. pp. 211-215.
To share on other social networks, click on any share button. What are these?








