Assessing the Impact of Insect Pest Biodiversity on the Ecological Footprint of Okra (Abelmoschus esculentus L.) Cultivation in Faisalabad, Punjab, Pakistan
Assessing the Impact of Insect Pest Biodiversity on the Ecological Footprint of Okra (Abelmoschus esculentus L.) Cultivation in Faisalabad, Punjab, Pakistan
Muhammad Ehsan Maqbool1*, Muhammad Nadeem Mushtaq1, Tamathues2, Khadija Tul Kubra1, Arish Zulfiqar1, Muhammad Shoaib Sarwar1, Zeeshan Yousaf3, Mudassar Yaseen1, Farwa Nosheen3
1Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad, Punjab, Pakistan
2Department of Entomology, PMAS Arid Agriculture University Rawalpindi, Punjab, Pakistan
3Department of Zoology, Government College University Faisalabad, Punjab, Pakistan
Abstract | In Pakistan, the main cause of poor production of okra (Abelmoschus esculentus L.) is due to different insect pests. The current study was conducted in Faisalabad, Punjab Pakistan to evaluate the diversity of different insect species in the okra crop. A total of 2613 specimens belonging to 19 species and 6 orders were collected with different methods. Most species (9) belong to the order Hemiptera, four species from Lepidoptera, three species from Coleoptera, and one species each from Diptera, Orthoptera, and Thysanoptera. Amrasca biguttula biguttula (31.38%) was dominant species throughout the study period followed by Aphis gossypii (12.39%), Podagrica sp. (10.98%), Bemisia tabaci (9.45%), Syllepte derogate (8.30%), Mylabris pustulata (7%), Phenacoccus solenopsis (4.17%), Thrips tabaci (3.52%), Dysdercus cingulatus (3.44%), Earias vittella (1.91%), Helicoverpa armigera (1.53%), Liriomyza trifolii (1.37%), Spodoptera litura (0.91%), Ricania speculum (0.84%), Menida histrio (0.76%), Nezara viridula (0.61%), Monolepta signata (0.57%), Atractomorpha crenulata (0.49%), Leptocentrus taurus (0.42%). Applying the shannon and simpson diversity index revealed notable variations throughout the okra crops. Such investigations are required in order to build integrated pest management (IPM) programs to control these economically important pests.
Novelty Statement | The current study represents the relationship between insect pest diversity and sustainable growth of the okra crop. It also fills a critical study gap by examining the impact of insect pests on this crop and promoting the development of new pest control techniques.
Article History
Received: December 30, 2023
Revised: July 25, 2024
Accepted: August 08, 2024
Published: October 11, 2024
Authors’ Contributions
MEM performed experiment and data curation. MNM and T planned methodology. KK and AZ analyzed the data. MSS and ZY wrote and reviewed the manuscript. MY and FN helped in formatting the manuscript.
Keywords
Bovine pituitary extract, Local rabbit, Biodiversity, Insects, Okra, Sucking pest, Chewing pest, Integrated Pest Management
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding Author: Muhammad Ehsan Maqbool
mianehsan164@gmail.com
To cite this article: Maqbool, M.E., Mushtaq, M., Tamathues, Kubra, K.T., Zulfiqar, A., Sarwar, M.S., Yousaf, Z., Yaseen, M. and Nosheen, F., 2024. Assessing the impact of insect pest biodiversity on the ecological footprint of okra (Abelmoschus esculentus L.) cultivation in Faisalabad, Punjab, Pakistan. Punjab Univ. J. Zool., 39(2): 169-175. https://dx.doi.org/10.17582/journal.pujz/2024/39.2.169.175
Introduction
Agriculture is the major source of revenue for about 75 percent of the world’s poor inhabitants. Improving agriculture productivity in small-scale production is crucial for alleviating poverty and achieving food security. By 2050, the agriculture industry is expected to grow by 60% to meet rising demand from a growing population. This would require increased crop yield in response to climate change (FAO, 2014). Climate change has a significant impact on agriculture; it has been affecting production techniques both directly and indirectly. Climate change poses new obstacles for agricultural development, output, and efficiency. Climate Smart Agriculture (CSA) strategy is used to achieve sustainable agricultural expansion while considering climate change and investment conditions (Siddiqui et al., 2023).
It is now more important than ever to carry out research on biodiversity in agroecosystems in order to fully understand the complex interactions that exist between agricultural plants and insects (Barberi et al., 2010; Giron et al., 2018). Insects are the world’s largest and dominating group of animals, with 751,000 known species. They account for three-fourths of all living organisms all over the world (Majumder et al., 2013). Although over a million insect species have been identified, the estimates of the total number of insect species vary from two to ten million but most of them remain undiscovered (Samways, 2005).
Okra (A. esculentus L.) is a food vegetable from the Malvaceae family. It is also known as “Bhendi” in the subcontinent, “Krejaib Kheaw” in Thailand, “Bamia” in the Middle East, and “Ladyfinger” in England. It is rich in minerals, carbohydrates fiber, protein, fat, and phenols (Ndunguru and Rajabu, 2004). It is one of the most popular and extensively farmed vegetables in Pakistan. Pakistan is rated fifth among major okra-producing countries, contributing 2% to global production (FAO, 2006; Gulsen et al., 2007; Huang et al., 2007; Javed et al., 2009). Okra accounts for 1.2% of Pakistan’s total vegetable production. Punjab produces the most okra (57.3%), followed by Balochistan (15.5%), KPK (13.9%), and Sindh (13.3%) (Khokhar, 2014).
Okra production is poor owing to some challenges including the lack of high-quality seed, insect pests, disease infestation, and others problems (Rahman, 2012). Insect pests are one of the most critical contributors to lowering okra yield. Approximately 72 bug species have been identified in the okra crop by Rahman et al. (2013). Most damage is caused by a variety of insect pests, including Jassid, Amrasca biguttula biguttula; Aphid, Aphis gossypii; Whitefly, Bemisia tabaci; Shoot and fruit borer, Earias insulana, Earias vittella; Leaf roller, Sylepta derogat (Mandal et al., 2006; Mane et al., 2010).
The current study seeks to obtain first-hand information as well as catalog the principal insect pests found in okra crop field in Faisalabad Punjab, Pakistan. Using this knowledge, a sustainable approach to pest control and pesticide reduction may be implemented as an important element of any future planned Integrated Pest Management (IPM) strategy.
Materials and Methods
Study site
The current study was carried out over the course of three months, from May to July 2023, in Student Farm of the University of Agriculture Faisalabad, Pakistan. One acre of okra crop was selected. Faisalabad is located in central Punjab, between latitude 30° 31.5° north and longitude 73° 74° east, 184.4 meters above sea level, with a maximum average temperature of 50°C during summer and a lowest average temperature of -1°C during winter (Figure 1). The average rainfall was 400 mm whereas the maximum precipitation was observed in July and August. The soil’s environmental and climatic qualities make it suited for growing both Rabi (winter season crops sown from October-December), and Kharif crops (Rainy or monsoon crops sown September-October).
Insect sampling
For taking insect samples, sweeping nets (30 cm diameter ring, 1.5 mm mesh, 2m long rod) and hand-picking methods were used at random spots. Besides this, the visual counting approach was also used to estimate the overall number of sucking insect pests and their relative abundance (Garcia et al., 1982). Ethyl acetate vapors were used to kill the collected insects. Larger insects were dry preserved whereas smaller insects were preserved in 70% ethanol.
Identification of insects
The insects were classified into families based on morphological traits using identification keys supplied by Borror et al. (1989), and Morleyv (1913). Some web sources were also explored for identification. Some specimens’ genus and species designations were determined with the assistance of taxonomists.
Biodiversity indices
In all following formulas ni indicates the total number of individuals in a given sample and N indicates the total population of all species.
Measurement of relative abundance
The following formula was used to calculate the proportion of individuals of a specific species overall species:

Where, RA stands for relative abundance.
Simpson index
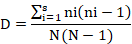
Shannon wiener index

Species evenness

H’, Shannon - Wiener index; S, Number of species in the community.
Species richness

S, Number of species in the community; N, Total population of all the species
Results and Discussion
The research studies were conducted from May to July 2023. A total of 2613 specimens belonging to six orders and 19 species were recorded from crops throughout the study period (Table 1, Figure 2). The evenness value indicated that the crop area was evenly distributed with only a few species of different orders dominating, namely Hemiptera (9 species), followed by Lepidoptera (4 species), and Coleoptera (with 3 species). One species each represented the remaining three orders, Diptera, Orthoptera, and Thysanoptera. The graphical representation in Figure 3 delineates the relative abundance of different insect orders.
A total of 732 individuals from 9 different species were collected in May from randomly chosen plants. The Jassid (A. biguttula biguttula), Cotton aphid (A. gossypii), Cotton mealybug (P. solenopsis), and White fly (B. tabaci) were the most common pests, with relative abundances of 30.7%, 12.7%, 8.9%, and 4.8%, respectively (Table 1).
Table 1: Different insect pest associated with okra in Faisalabad.
|
S. No |
Family |
Common name |
Scientific name |
Relative abundance |
Part infected |
||
|
May |
June |
July |
|||||
|
Hemiptera |
|||||||
|
1 |
Cicadellidae |
Jassid |
Amrasca biguttula biguttula |
30.7 |
27.9 |
35.9 |
Leaf |
|
2 |
Aleyrodidae |
White fly |
Bemisia tabaci (Gennadius) |
8.9 |
9 |
10.6 |
Leaf |
|
3 |
Aphididae |
Cotton aphid |
Aphis gossypii (Glover) |
12.7 |
12 |
12.9 |
Leaf, flower buds, flowers |
|
4 |
Pyrrhocoridae |
Red Cotton Bug |
Dysdercus cingulatus (Fabricius) |
3.6 |
3.9 |
3.1 |
Leaf, shoot and fruit |
|
5 |
Pseudococcidae |
Cotton mealybug |
Phenacoccus solenopsis (Tinsley) |
4.8 |
3.2 |
4.4 |
Leaf, fruit |
|
6 |
Pentatomidae |
Green stink bug |
Nezara viridula (Linnaeus) |
0.4 |
0.5 |
0.9 |
Leaf, shoot and fruit |
|
7 |
Pentatomidae |
Shield bug |
Menida histrio (Fabricius) |
0.5 |
0.6 |
1.1 |
Buds, fruits |
|
8 |
Ricaniidae |
Black leafhopper |
Ricania speculum (Walker) |
0.7 |
0.8 |
1 |
Leaf, stem |
|
9 |
Membracidae |
Cow bug |
Leptocentrus taurus (Fabricius) |
0.1 |
0.7 |
0.4 |
Leaf, stem |
|
Lepidoptera |
|||||||
|
10 |
Noctuidae |
American boll worm |
Helicoverpa armigera (Hübner) |
0.0 |
1.7 |
2.6 |
Fruit |
|
11 |
Noctuidae |
Tobacco caterpillar |
Spodoptera litura (Fabricius) |
0.7 |
0.9 |
1.1 |
Leaf |
|
12 |
Nolidae |
Shoot and fruit borer |
Earias vittella (Fabricius) |
0.0 |
2.8 |
2.9 |
Shoot, fruit |
|
13 |
Crambidae |
Cotton leaf roller |
Syllepte derogata (Fabricius) |
7.9 |
8.3 |
8.8 |
Leaf |
|
Coleoptera |
|||||||
|
14 |
Chrysomelidae |
White-spotted leaf beetle |
Monolepta signata (Olivier) |
0.7 |
0.8 |
0.3 |
Leaf, flower |
|
15 |
Chrysomelidae |
Leaf eating beetle |
Podagrica sp. |
10.7 |
10.6 |
11.9 |
Leaf |
|
16 |
Meloidae |
Blister beetle |
Mylabris pustulata (Thunberg) |
11.1 |
11.6 |
0.0 |
Flower buds and flower |
|
Diptera |
|||||||
|
17 |
Agromyzidae |
Serpentine leafminer |
Liriomyza trifolii (Burgess) |
1.2 |
1.7 |
1.2 |
Leaf |
|
Orthoptera |
|||||||
|
18 |
Pyrgomorphidae |
Tobacco grasshopper |
Atractomorpha crenulata (Fabricius) |
0.7 |
0.3 |
0.5 |
Leaf |
|
Thysanoptera |
|||||||
|
19 |
Thripidae |
Onion thrips |
Thrips tabaci (Linderman) |
4.6 |
3.1 |
3.1 |
Leaf |
However, it was discovered that the cotton mealybug (P. solenopsis) and onion thrips (T. tabaci) were the two main pests inflicting significant damage during the first month. Onion thrips adults have been seen to eat leaves in large quantities. It was discovered that cotton mealybug adults and nymphs infested leaves and young shoots. As a result of feasting on sap, the shoots developed certain distinctive marks that rendered them unmarketable (Ansari at al., 2019).
During June the Blister beetle (M. pustulata), Red Cotton Bug (D. cingulatus), and Serpentine leafminer (L. trifolii) were the major pests with a relative abundance of 11.6%, 3.9%, and 1.7%, respectively. Blister beetles attack flower buds and fruit settings while the Red Cotton Bug damages tissues which are then colonized by microorganisms that cause boll rot and discoloration. Boll abortion, premature opening, and early shed are common. Further symptoms are smaller seeds with reduced oil content, stained fibers, and lower germination rate. These seeds are not fit for sowing. Serpentine leafminer particularly eats the leaf and causes distinctive mine damage in okra.
All kinds of insect pests attack during July because there is an abundance of leaves, flowers, bolls (small green triangular pods or capsule of some plants), and fruits accessible. The primary pests for this month were recorded as cotton Jassid 35.9%, cotton mealybug 4.4%, shoot and fruit borer 2.9%, and leaf-eating beetle 11.9%. Jassid drained the leaves of their juice, causing harm. Plants that are widely infected may become severely damaged and develop poorly because of the vast amounts of sap that may be withdrawn, mostly by the developing nymphs. Frequently, leaves get yellow, seem dry, and fall off too soon. In addition to slowing down okra’s growth, cotton aphids spread illness (Shabozoi et al., 2011). Significant cell fluid loss causes a shortened life span and stunts the development of the plant. Significant local damage was caused by the leaf roller. The severely attacked okra plants had all of their foliage removed, which inhibited the plants development and caused the bolls to ripen too soon, reducing production (Singh et al., 2013).
Shoot and fruit borer larvae bore into delicate terminal stems during the vegetative stage and flower buds, flowers, and early fruits during the fruit development stage. The broken shoots droop, dry out and wither. The contaminated fruits have a malformed look and are no longer appropriate for eating. Leaf-eating beetles cause holes in the leaves of okra which decrease the area for photosynthesis (Rather et al., 2021).
Shannon-Weiner diversity index was used to calculate diversity, species richness, and species evenness The insect pest complex of okra observed almost equal diversification over the course of the research period, as indicated by the computed Shannon and Wiener diversity index (H’) values for May, June, and July are 2.18, 2.23, and 2.16, respectively. In the okra ecosystem, there is a high degree of variety among insect pest species in July, followed by May and June. This is shown by the Simpson’s diversity index (D) for May, June, and July, which was computed as 0.15, 0.13, and 0.17, respectively. Likewise, for each of the three months, the Species Evenness values (0.769, 0.789, and 0.750) were roughly identical (Table 2).
Table 2: Shows the diversity, evenness, and richness of insect pest complexes in okra throughout a three-month study period.
|
Month |
No of species |
Total no of individuals |
Shannon index |
Simpsons index |
Species evenness |
Species richness |
|
May |
17 |
732 |
2.18 |
0.15 |
0.769 |
28.01 |
|
June |
19 |
878 |
2.32 |
0.13 |
0.789 |
33.60 |
|
July |
18 |
1003 |
2.16 |
0.17 |
0.750 |
38.38 |
The highest number of species attacked occurred in June, totaling 19 species with a species richness of 33.60. This is the peak growth season for the okra crop in Punjab Pakistan, and plants have increased in biomass, making them more sensitive to several insect pests. It is a traditional vegetable crop of Pakistan, grown during the Kharif and Rabi seasons. It is commercially well established in local trade and is mostly handled by small farmers. It is a garden crop that is grown economically and commercially and is utilized all over the world (Arapitsas, 2008; Saifullah and Rabbani, 2009; Akbar et al., 2012). Poor and ineffective pest and disease control is a severe restriction in vegetable production, resulting in high yield losses (Tindall, 1983).
July has the lowest overall number of species, but the highest number of insects captured, making it the most species rich month of the study with 38.38 species richness. Small-scale farmers suffer a significant reduction in revenue due to this, which can reach up to 25 percent (Chaudhary and Dadheeck, 1989). Increased eating of fruits and vegetables is a global goal for preventing the world’s most common and devastating nutritional diseases. This, however, necessitates better production as well as distribution networks, both of which pose significant hurdles (FAO, 2013). A. esculentus L. is one of the major vegetables in tropical regions, which is used raw or cooked in salads, soups, and stews. Its soft fruit is rich in carbohydrates, vitamins A and C, and other minerals including calcium, magnesium, iron, and phosphorus (Kashif et al., 2008).
The lowest species caught occurred in May, with 17 species and a total of 732 individuals. It is the least concerned month of the study, with 28.01 species richness. Sabyasachi et al. (2013) identified over 72 insect species on okra crop. The current study found that the insect fauna in okra crops was diversified at all taxonomic levels. Their population shifted with the different stages of crop. In all of the research months, insects were prevalent from May to July. Temperatures remain favorable during these months, while plants and weeds grow in the agro environments due to monsoon rain. The abundance of nectar and host insects enhanced the population of insects. Even while the overall number of families did not fluctuate dramatically between study months, the total number of species did. It has been estimated that around 54% of okra losses may have been prevented by pests control (Chaudhary and Dadheeck, 1989).
The present results are in accordance with the findings of Obeng-Ofori and Sackey (2003) identified 18 insect species in the okra environment. According to Mandal et al. (2006), A. esculentus L. was destroyed by a variety of pests including Lepidoptera, Homoptera, Coleoptera, Orthoptera, and Thysanoptera. Furthermore, 112 insect species (Chakraborty et al., 2014), 28 species (Nair et al., 2017), and 17 species of insects (Bhatt et al., 2018) have been reported in the okra environment. Amin et al. (2019) identified 29 species of insect pests in okra.
Conclusions and Recommendations
The study concludes that there are fluctuations in the population density of different pest species during the study period. Sucking pests initially take over fields during the early phenological stage of the crop. Later, when the plants go through the subsequent phenological stages, chewing pests take over the fields. It is crucial to control insect pests from okra crop by using IPM approach. The pest species should be controlled by species-specific pest monitoring by using different traps, pheromones and visual or live counting. Cultural techniques including crop rotation, intercropping and pest repellent crops should be adopted. In biological control natural enemies of insect pest must be introduced in crop. Moreover, community participation plays a pivotal role to raise awareness among farmers.
Declarations
Acknowledgment
The authors are thankful to the Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad for providing research facilities.
Funding
This research did not receive any funding.
IRB approval
The experiment was carried out in-line with the IRB Guidelines of University of Agriculture, Faisalabad.
Ethical statement
All the procedures and methods used in this study followed the ethical guidelines provided by University of Agriculture, Faisalabad.
Data availability statement
Data will be available on request.
Conflicts of interest
The authors have declared no conflict of interest.
References
Akbar M.F., Haq, M.A. and Yasmin, N., 2012. Effectiveness of bio-insecticides as compared to conventional insecticides against jassid (Amrasca devastans Dist.) on okra (Abelmoschus esculentus L.) crop. Pak. Entomol., 34: 161-165.
Amin, M.R., Tripura, K., Miah, M.R.U., Kayesh, E. and Khan, M.A.R., 2019. Diversity of insects in okra agro-ecosystem at Gazipur in Bangladesh. Indian J. Ecol., 46: 214-216.
Ansari, M.S., Basri, R. and Shekhawat, S.S., 2019. Insect pests infestation during field and storage of fruits and vegetables. Hlth. Saf. Asp. Fd. Process. Technol., pp. 121-207. https://doi.org/10.1007/978-3-030-24903-8_7
Arapitsas, P., 2008. Identification and quantification of polyphenolic compounds from okra seeds and skins. Fd. Chem., 110: 1041-1045. https://doi.org/10.1016/j.foodchem.2008.03.014
Barberi, P., Burgio, G., Dinelli, G., Moonen, A.C., Otto, S., Vazzana, C. and Zanin, G., 2010. Functional biodiversity in the agricultural landscape: relationships between weeds and arthropod fauna. Weed Res., 50: 388-401. https://doi.org/10.1111/j.1365-3180.2010.00798.x
Bhatt, B., Joshi, S. and Karnatak, A.K., 2018. Biodiversity of insect pests and their predators on okra agroecosystem. J. Pharmacogn. Phytochem., 7: 84-86. https://doi.org/10.20546/ijcmas.2018.709.091
Borror, D.J., Triplehorn, C.A. and Jonson, N.F., 1989. An introduction to the study of insects, 6th Edition, Saunders College Publishing, USA.
Chakraborty, A., Kumar, K. and Rajadurai, G., 2014. Biodiversity of insect fauna in okra (Abelmoschus esculentus (L.) Moench) ecosystem. Trends Biosci., 7: 2206-2211.
Chaudhary, H.R. and Dadheeck, L.N., 1989. Incidence of insects attacking okra and the avoidable losses by them. Ann. Arid Zone., 28: 305-307.
FAO, 2006. The state of food and agriculture. Food and Agriculture Organization of the United Nations.
FAO, 2013. The state of food and agriculture. Innovative food systems. Rome.
FAO, 2014. The state of food and agriculture. In: The State of Food and Agriculture, UN.
Garcia, A., Gonzalez, D. and Leigh, T.F., 1982. Three methods for sampling arthropod numbers on California cotton. Environ. Entomol., 11: 565-572. https://doi.org/10.1093/ee/11.3.565
Giron, D., Dubreuil, G., Bennett, A., Dedeine, F., Dicke, M., Dyer, L.A., Erb, M., Harris, M.O., Huguet, E., Kaloshian, I., Kawakita, A., Lopez-Vaamonde, C., Palmer, T.M., Petanidou, T., Poulsen, M., Sallé, A., Simon, J.-C., Terblanche, J.S., Thiéry, D., Whiteman, N.K., Woods, H.A. and Pincebourde, S., 2018. Promises and challenges in insect plant interactions. Entomol. Exp. appl., 166: 319-343. https://doi.org/10.1111/eea.12679
Gulsen, O., Karagul, S. and Abak, K., 2007. Diversity and relationships among Turkish okra germplasm by SRAP and phenotypic marker polymorphism. Biol. Brat., 62: 41-45. https://doi.org/10.2478/s11756-007-0010-y
Huang, Z., Wang, B., Eaves, D.H., Shikany, J.M. and Pace, R.D., 2007. Phenolic compound profile of selected vegetables frequently consumed by African Americans in the southeast United States. Fd. Chem., 103: 1395-1402. https://doi.org/10.1016/j.foodchem.2006.10.077
Javed, H., Aziz, M.A. and Leghari, R.A.K., 2009. Resistance in different okra cultivars (Abelmoschus esculetus L.) against American bollworm (Helicoverpa armigera Hübner). J. Agric. Res., 47: 433-438.
Kashif, S., Yaseen, M., Arshad, M. and Ayub, M., 2008. Response of okra (Hibiscus esculentus L.) to soil given encapsulated calcium carbide. Pak. J. Bot., 40: 175-181.
Khokhar, K.M., 2014. Production status of major vegetables in Pakistan, their problems and suggestions. National Agricultural Research Council (NARC), Islamabad, Pakistan.
Majumder, J., Das, R.K., Majumder, P., Ghosh, D. and Agarwala, B.K., 2013. Aquatic insect fauna and diversity in urban fresh water lakes of Tripura, Northeast India. Middle East J. Sci. Res., 13: 25-32.
Mandal, S.K., Sah, S.B. and Gupta, S.C., 2006. Screening of okra cultivars against Earias vitelli. Ann. Pl. Prot. Sci., 14: 471-472.
Mane, S.A., Waghmare, U.M. and Yadav, G.A., 2010. Bio-Efficacy and economoics of some newer insecticides against fruit borer (Earias vitella Fabricius) of okra. Pestology, 34: 101-104.
Morley, C.I., 1913. The fauna of British India including Ceylon and Burma. Hymenoptera III, Taylor and Francis, London, UK, pp. 1-531.
Nair, N., Giri, U., Bhattacharjee, T., Thangjam, B., Paul, N. and Debnath, M.R., 2017. Biodiversity of insect pest complex infesting okra (Abelmoschus esculentus) in Tripura, NE India. J. Entomol. Zool. Stud., 5: 1968-1972.
Ndunguru, J. and Rajabu, A.C., 2004. Effect of okra mosaic virus disease on the above-ground morphological yield components of okra in Tanzania. Sci. Hortic., 99: 225-235. https://doi.org/10.1016/S0304-4238(03)00108-0
Obeng-Ofori, D. and Sackey, J., 2003. Field evaluation of non-synthetic insecticides for the management of insect pests of okra Abelmoschus esculentus (L.) Moench in Ghana. Sinet: Ethiop. J. Sci., 26: 145-150. https://doi.org/10.4314/sinet.v26i2.18210
Rahman, K., 2012. Performance of different okra (Abelmoschus esculentus L.) cultivars under the agro-climatic conditions of Dera Ismail Khan. Pak. J. Sci., 64: 316-319.
Rahman, M.M., Uddin, M.M. and Shahjahan, M., 2013. Management of okra shoot and fruit borer, Earias vittella (Fabricius) using chemical and botanical insecticides for different okra varieties. Int. Res. J. app. Life Sci., 2: 1-9.
Rather, B.A., Mir, M.M., Iqbal, U. and Mir, S.A., 2021. Integrated pest management of stone fruits, production technology of stone fruits. pp. 397-422. https://doi.org/10.1007/978-981-15-8920-1_15
Sabyasachi, S.P., Maji, T.B. and Palash Mondal, P.M., 2013. Incidence of insect pest on okra, Abelmoschus esculentus (L) Moench in red lateritic zone of West Bengal. J. Plant Prot. Sci., 5: 59-64.
Saifullah, M. and Rabbani, M.G., 2009. Evaluation and characterization of okra (Abelmoschus esculentus L. Moench.) genotypes. SAARC. J. Agric., 7: 92-99.
Samways, M.J., 2005. Insect diversity conservation. Cambridge University Press, Cambridge, UK, pp. 342. https://doi.org/10.1017/CBO9780511614163
Shabozoi, N.U.K., Abro, G.H., Syed, T.S. and Awan, M.S., 2011. Economic appraisal of pest management options in okra. Pakistan J. Zool., 43: 869-878.
Siddiqui, A.O., Naeem, M.Y. and Selamoglu, Z., 2023. Climate smart agriculture and food security. Int. J. Environ. Res. Educ., 3: 27-34.
Singh, Y., Jha, A., Verma, S., Mishra, V.K. and Singh, S.S., 2013. Population dynamics of sucking insect pests and its natural enemies on okra agro-ecosystem in Chitrakoot region. Afr. J. Agric. Res., 8: 3814-3819. https://doi.org/10.5897/AJAR12.1743
Tindall, H., 1983. Vegetables in the tropics. Oxford Press, London. pp. 90-145. https://doi.org/10.1007/978-1-349-17223-8
To share on other social networks, click on any share button. What are these?








