Bio-Efficacy of Neonicotinoids and Insect Growth Regulators against Bemicia tabaci Genn. on Solanum Melongena L.
Bio-Efficacy of Neonicotinoids and Insect Growth Regulators against Bemicia tabaci Genn. on Solanum Melongena L.
Muhammad Shoaib Saleem* and Muhammad Faheem Akbar
Department of Agriculture and Agribusiness Management, University of Karachi, Karachi
ABSTRACT
Recently, great attention has been paid towards exploitation of Bio-rational insecticides in plant pest management in perspective of the public concern over the level of pesticide residues in food and environment causing health and ecological problems. The indiscriminate use of synthetics insecticides in crop protection has also led to development of resistant strains of pests and have adverse effects on non-target organisms including natural enemies and pollinators. Amongst bio-rational insecticides, neonicotinoids and insect growth regulators (IGRs) may be exploited as eco-friendly approach in crop pest management. We therefore, evaluated the effectiveness of nitenpyram, Clothianidin, Momentum (Mixture of Nitenpyram and Chlorfenapyr) and Buprofezin against brinjal whitefly (Bemisia tabaci Genn.). The crop was grown in a randomized complete block design (RCBD) with three replicates each having five treatments with control. Pre-treatment data were collected before 24 h and Post treatment data were collected after 24, 72, 168 and 336 h of each spray. In this manner, the data for three sprays were collected. The order of effectiveness in decreasing sequence was found to be Nitenpyram, Buprofezin, clothianidin and momentum (75%), (65%) (64%) and (63%), respectively against whiteflies’ population.
Article Information
Received 27 May 2018
Revised 20 June 2018
Accepted 25 June 2018
Available online 10 June 2019
Authors’ Contribution
MFA conceived and designed the study. MSS conducted experiments, collected and analyzed the data and wrote the article.
Key words
Bemicia tabaci (Genn.), Bio efficacy, Insect growth regulators (IGRs), Neonicotinoids, Selected insecticides, Brinjal.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.5.1615.1620
* Corresponding author: shoaibsaleemkhan@gmail.com
0030-9923/2019/0005-1615 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Brinjal (Solanum melongena L.) belongs to the family solanacea and one of the most widely cultivated crops around the world (EGGNET, 2005). It is the most important vegetable crop grown in Asia, Africa and Mediterranean countries (Collonier et al., 2001). In Pakistan the cultivated area under brinjal crop has been reported 8325 hectares with an annual production of 82999 tonnes and the average yield 9969.8 Kg/ha (FAOSTAT, 2017). Different cultivars of Brinjal grown in Pakistan, contain the wide range of fruit shapes ranging from oval, egg-shaped to long club-shaped with white, yellow, green, purple pigmentation to almost black colors. Brinjal is adapted to high rainfall and high temperature. Besides that, it has potential to produce high yield under hot-wet environment (Peter et al., 2006). However, brinjal production effects due to the severe attack of sucking insect pests. Among these whitefly (Bemisia tabaci Genn.) is the most destructive one (Abhijit and Chatterjee, 2012; Norhelina et al., 2013). Both nymphs and adults feed on the lower surface of the leaves and suck the sap from sieve tubes. They also secret the honey dew, thus lower down photosynthetic activity of the plants (Khan et al., 2011).Whitefly acts as a vector for some pathogens and viruses that cause damage to the plant (Fauziah et al., 2009). An estimated 70% to 92% yield losses has been reported due to whitefly infestation (Omprakash and Raju, 2014).
Although insecticidal application is the most common practice to control the whitefly, but the toxic nature of insecticides and their continuous and injudicious use are harmful for the human health and the environment as well. As the vegetable crops are consumed just after few days of harvest, hence, there is always a risk of exceeding MRLs, which is ultimately hazardous to human health. Continuous intake of insecticide residues even in trace elements may accumulated in human body tissues and cause severe health effects (Handa et al., 1999). It was also confirmed by Akbar et al. (2010a, b, 2012a) that the residues of Organochlorine and Organophosphate insecticides in different vegetables are higher than maximum residual limits (MRLs) set by Codex (FAO) and EU. Moreover, Masud and Hasan (1992) also reported higher levels of insecticides residues in different vegetables.
In Pakistan, management of whitefly (Bemisia tabaci Genn.) is much relies on conventional insecticides. Continuous and injudicious application of chemical have also developed resistance to conventional insecticides. Neonicotinoids are the new class of insecticides with novel mode of action, broad spectrum, good trans laminar ability, long systemic activity (Kodandaram et al., 2010). Selectivity, lower dose and safer to non-target organisms is very appropriate in integrated pest management (IPM) and insect resistance management (IRM) resulting in less harmful for environment. On the other hand insect growth regulators (IGRs) were also found most effective against nymphal stages of whitefly (Nadeem et al., 2011; Ramalakshmi et al., 2012).
Keeping in view the significance of export and economic value of the brinjal crop and other threats to environment and non-target organisms by broad spectrum insecticides; present study was planned to evaluate the effectiveness of neonicotinoids and IGRs against whitefly on brinjal crop.
Materials and Methods
Experimental sites
Experiments were conducted at the agricultural Field of Department of Agriculture and Agribusiness Management, University of Karachi during 2016-17.
Plant materials
A local Variety “Black King” F1 hybrid brinjal was used as a host for the pest. Seeds were obtained from local market. The brinjal seeds were sown in small pots. After 35 days, the seedlings were transplanted to the field with a row to row distance of 75 cm and plant to plant distance of 60 cm and
Field experiment layout and design
The experimental plots were arranged in a randomized complete block design (RCBD), with three replicates, each comprising five treatments including control.
Application of insecticides and their specifications
The insecticides (Table I) were applied at prescribed dose mentioned on the products’ label when the population of whitefly reached at economic threshold level (ETL) i.e. 5 to 10 whiteflies per leaf (Shivanna et al., 2011).
Data collection
The observations of whitefly were recorded on ten randomly selected plants from each treated plot. Three leaves from each plant were observed from top, middle and bottom (Kaushik and Kaushik, 1990). The whitefly population was carefully counted on under side of the leaves. Finally, the collected data were expressed as mean populations from each plot. Pre-treatment counts were taken before 24 h, while post-treatment observations were made after 24, 72, 168 and 336 h of each spray.
The obtained data were compiled as a mean populations and reduction percentage was calculated by Henderson-Tilton’s formula (Henderson and Tilton, 1955) according to following equation:
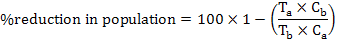
Where, Ta is number of insects after treatment, Tb is number of insects before treatment, Ca is number of insects in untreated check after treatment and Cb is number of insects in untreated check before treatment.
Statistical analysis
The collected data were subjected to statistical analysis through SPSS version 16.0. The mean differences between various treatments were tested by using Tukey’s HSD test at 5% significance Level.
Results and Discussion
The results in Table II reveals that all the treatments had significant effect (P<0.05) against whiteflies’ population even till 14 days.
After 24 h of first spray, maximum reduction in whitefly population was recorded in the plots treated with Nitenpyram (70.02%), Clothianidin (57.95%) and Momentum (Nitenpyram + Chlorfenapyr) (57.25%), while Buprofezin gave minimum reduction with (41.20%). After three days, maximum reduction was recorded as nitenpyram (78.41%), clothianidin (69.63%) and momentum (64.86%), followed by Buprofezin (54.52%). While, after seven days, nitenpyram gave (68.98%) reduction and Buprofezin (64.89%) followed by clothianidin (63.64%) and momentum (59.41%), which were significantly different (P<0.01) as compared to control. However, after 14 days, the maximum reduction was recorded in the plots treated with Buprofezin (66.08%) and clothianidin (64.10%), followed by nitenpyram (63.04%) and momentum (61.19%).
Table I.- Insecticides used against whitefly on brinjal crop.
|
S. No. |
Common name |
Trade name |
Group |
Source |
Dose g ha-1 a.i. |
|
1. |
Nitenpyram |
Pyramid 10% SL |
Neonicotinoids |
Kanzo AG |
49.4 |
|
2. |
Clothianidin |
Telsta |
Neonicotinoids |
Sun Crop |
24.7 |
|
3. |
Nitenpyram + Chlorfenapyr |
Momentum 50% WDG |
Neonicotinoids and Pyrrole |
Kanzo AG |
7.41 |
|
4. |
Buprofezin |
Applaud |
IGRs |
Arysta |
59.28 |
Table II.- Mean and percentage reduction of whitefly populations on brinjal leaves after applications of different insecticides.
|
Treatments |
Mean and reduction percentage of whiteflies (Bemisia tabaci) per 10 leaves |
Commutative mean |
Percent reduction over control |
|||
|
24 h |
72 h |
168 h |
336 h |
|||
|
First spray |
||||||
|
Nitenpyram |
9.00a (70.02) |
5.33a (78.41) |
3.66a (83.89) |
9.66a (63.04) |
6.87a |
73.85 |
|
Clothianidin |
15.66bc (57.95) |
11.33bc (69.63) |
1.33a (63.34) |
12.00a (64.10) |
12.80ab |
63.76 |
|
Momentum |
9.66a (57.25) |
8.00bc (64.86) |
8.66a (59.41) |
8.33a (61.19) |
8.62a |
60.68 |
|
Buprofezin |
21.66b (41.20) |
16.66b (54.52) |
11.66a (64.89) |
12.33a (66.08) |
15.52b |
56.67 |
|
Control |
42.33a (0.00) |
43.30c (0.00) |
39.00b (0.00) |
40.00b (0.00) |
41.15c |
- |
|
Second spray |
||||||
|
Nitenpyram |
4.00a (82.89) |
3.66a (79.82) |
5.33a (77.49) |
8.00a (71.99) |
5.22a |
78.05 |
|
Clothianidin |
11.00a (65.00) |
9.00a (71.04) |
11.66a (61.39) |
15.33a (58.81) |
11.72b |
64.06 |
|
Momentum |
6.33a (64.92) |
7.33a (60.52) |
7.33a (63.12) |
9.00a (59.19) |
7.47ab |
61.94 |
|
Buprofezin |
11.66a (58.89) |
9.66a (66.15) |
9.66a (68.31) |
11.00a (70.40) |
10.45b |
65.94 |
|
Control |
36.33a (0.00) |
34.66b (0.00) |
35.66b (0.00) |
42.33b (0.00) |
37.20c |
- |
|
Third spray |
||||||
|
Nitenpyram |
16.00a (75.51) |
13.00a (80.92) |
5.33a (85.94) |
5.33a (81.75) |
9.00a |
81.03 |
|
Clothianidin |
27.00a (65.47) |
25.66a (68.15) |
19.00a (67.24) |
11.66a (60.67) |
20.95a |
65.38 |
|
Momentum |
18.00a (65.15) |
15.33a (68.78) |
8.66a (71.27) |
8.00a (58.40) |
12.47a |
65.90 |
|
Buprofezin |
26.33a (68.69) |
16.33a (81.44) |
16.00a (74.94) |
10.00a (69.14) |
17.15a |
73.55 |
|
Control |
96.66b (0.00) |
96.00b (0.00) |
68.33b (0.00) |
37.33b (0.00) |
74.55b |
- |
*, means sharing similar alphabets in each column are not significantly difference (Tukey‟s HSD, P > 0.05). **, values in parenthesis represent percent reduction of whiteflies in each treatment.
Similar trend was observed after 2nd spray. Nitenpyram maintained its superiority over rest of insecticides with an increasing trend with 82.89%, 79.82%, 77.49% and 71.99% reduction in whitefly population after 1, 3, 7 and 14 days of 2nd spray, while effectiveness decreased after 7 days of spray as the time increased. Although clothianidin and momentum (Nitenpyram+Chlorfenapyr) were effective with 65.00%, 71.04%, 61.39% and 58.81%, and 64.92%, 60.52%, 63.12% and 59.19% reduction, respectively. Buprofezin performed well as compared to 1st spray with 58.89%, 66.15%, 68.31 and 70.40% insect mortality.
Nitenpyram sustained its dominance with increasing trend till 3rd spray and reduced whitefly population by 75.51%, 80.92%, 85.94% and 81.75%. Clothianidin and Momentum gave similar results with gradual decrease in effectiveness as compared to previous treatments with 65.47%, 68.15%, 67.24% and 60.67%, and 65.15%, 68.78%, 71.27% and 58.40% insect mortality. However, buprofezin gave satisfactory results with gradual increasing trend in effectiveness with 68.69%, 81.44%, 74.94% and 69.14% reduction in jassid population. Buprofezin showed higher mortality of whiteflies at third and second spray till 14 days.
After three consecutive sprays, an overall performance of all the insecticides represents that nitenpyram was highly effective against whiteflies, followed by buprofezin, clothianinidin and momentum (Nitenpyram+Chlorfenapyr) 75±4.5, 65±6.9, 64±0.4 and 63±2.2, respectively.
The variable toxic effects against whiteflies, might be due to different characteristics of neonicotinoids which influence the movement in plant tissues such as solubility of the water that greatly affects the toxicity in sucking and piercing type insects like whiteflies (Cloyd-Raymond and Bethke, 2011). The tested neonicotinoids insecticides showed inconsistent reduction of whitefly population during three sprays in the similar cropping season. Based on the results, it was observed that the reduction percentage of whitefly population is higher in first spray than the second and third sprays. This may be due to the surrounding temperature and high intense sunlight that reduced toxicity of insecticides compounds. These findings are supported by the Wei and Liu (1999), who reported that hydrolysis of neonicotinoids insecticides increases with the increased surrounding temperature, which affects the toxicity level of insecticides.
The findings of the present study showed that nitenpyram was effective with maximum mortality till 14 days as compared to rest of insecticides, which is in consistence with the findings of Amit and Raghuraman (2014), they reported that nitenpyram significantly reduced the whiteflies population in vegetable crops. This was also confirmed that Asif et al. (2017), neonicotinoids i.e. nitenpyram are very effective in reducing the population of whitefly below economic threshold level. Moreover, Irshad et al. (2015) observed that nitenpyram reduced the sucking insects population below ETL after seven days of application. Whereas, Kadam et al. (2014) found nitenpyram significantly effective against sucking insects over a span of 14 days. Clothianidin proved its effectiveness after nitenpyram against brinjal whitefly, which confirms the similar studies conducted by Vijay and Ilyas (2017), Patnaik et al. (2011) and Shaikh et al. (2014). It was also found by Pachundkar et al. (2013) that the higher effectiveness of clothianidin 50 WDG (0.025%) against the whitefly. These findings also supported by Akbar et al. (2008, 2010a, 2014) that imidacloprid (neonicotinoid) most effective against Myzus persicae (Sulzer) on mustard, cabbage and cauliflower as compared to endosulfan. They also reported its excellent efficacy against Bemicia tabaci Genn. on okra and brinjal (Akbar et al., 2009, 2011, 2015a) and Amrasca devastans Distt. on potato, okra and brinjal (Akbar et al., 2012a, b, 2015b). Momentum (a mixture of nitenpyram and chlorfenapyr) showed moderate effectiveness during the present study, that is in the line of previous findings that confirms its good performance against whitefly till one week after application (Anonymous, 2016).
Buprofezin significantly reduced the whiteflies’ population after nitenpyram and clothianidin. These findings are in conformity with Maji et al. (2015), who reported buprofezin as highly effective against sucking insects of okra. Nadeem et al. (2011) found that buprofezin was the most effective insecticides against nymphs of whitefly in Pakistan. Ramalakshmi et al. (2012) found buprofezin 25% SC significantly effective in reducing leafhopper (A. devastans) population on cotton crop. Gopal and Islam (2014) also found that buprofezin was the best insecticide for the management of brinjal whitelfies. In another study, Amit and Raghuraman (2014) reported significant mortality of whitefly through buprofezin. This is due to the mode of action of buprofezin that once whitefly poisoned, they become unable to produce new cuticle, thereby effectively preventing them from molting to the next stage and finally they die due to poisoning of buprofezin.
Figure 1A presents the time wise effectiveness of all four tested insecticides. Increasing trend was observed in all the insecticides till 336 h post application. Whereas, the spray wise efficacy of the tested insecticide (Fig. 1B) showed higher mortality of whitefly after the third spray while first and second spray showed less effectiveness.
Conclusion
It could be concluded that being bio rational, all the tested insecticides including Insect Growth Regulators (IGRs) can successfully be incorporated in an Integrated Pest Management (IPM) strategy. During the present study, Nitenpyram and Buprofezin has proved them most effective in suppressing whiteflies’ populations on brinjal crop.
Acknowledgement
Authors are thankful to Dean Faculty of Sciences, University of Karachi, for providing financial grant for carrying out this research work.
Statement of conflict of interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
Abhijit, G. and Chatterjee, M.L., 2012. Bioefficacy of imidacloprid 17.8 SL against whitefly, Bemisia tabaci (Genn.) in brinjal. J. Pl. Protec. Sci., 5: 37-41.
Akbar, M.F., Yasmin, N., Naz, F. and Haq, M.A., 2008. Efficacy of imidacloprid and endosulfan in comparison with biosal (Biopesticide) against Myzus persicae (sulzer) on mustard crop. Pak. J. Ent., 23: 27-30.
Akbar, M.F., Yasmin, N., Naz, F. and Latif, T.A., 2009. Effectiveness of different spray schedules against population of whitefly, Bemisia tabaci (Genn.) on okra crop. Pak. J. Ent., 24: 45-48.
Akbar, M.F., Haq, M.A., Parveen, F., Yasmin, N. and Khan, M.F.U., 2010a. Comparative management of cabbage aphid Myzus persicae (Sulzer) (Aphididae: Hemiptera) through bio-and synthetic-insecticides. Pak. Entomol., 32: 12-17.
Akbar, M.F., Haq, M.A., Parveen, F., Yasmin, N. and Sayeed, S.A., 2010b. Determination of synthetic and bio-insecticides residues during aphid, Myzus persicae (Sulzer) control on cabbage crop through high performance liquid chromatography. Pak. Entomol., 32: 155-162.
Akbar, M.F., Haq, M.A., Yasmin, N., Khan, M.F. and Azmi, M.A., 2011. Efficacy of bio-insecticides as compared to conventional insecticides against white fly (Bemisia tabaci Genn.) on okra crop. Pak. J. Ent., 26: 16-23.
Akbar, M.F., Haq, M.A., Yasmin, N. and Khan, M.F., 2012a. Degradation analysis of some synthetic and bio-insecticides sprayed on okra crop using HPLCi. J. chem. Soc. Pak., 34: 306-311.
Akbar, M.F., Haq, M.A. and Yasmin, N., 2012b. Effectiveness of bio-insecticides as compared to conventional insecticides against jassid (Amrasca devastans Dist.) on okra (Abelmoschus esculentus L.) crop. Pak. Entomol., 34: 161-164.
Akbar, M.F., Rana, H.U. and Perveen, F., 2014. Management of cauliflower aphid (Myzus persicae (Sulzer) (Aphididae: Hemiptera) through environment friendly bioinsecticides. Pak. Entomol., 36: 25-30.
Akbar, M.F., Haq, M.A., Rana, H.U. and Khan, M.F., 2015a. Role of bio-rational insecticides in controlling Amrasca devastans Dist. on Solanum melongena L. crop. Int. J. Biol. Biotech., 12: 401-406.
Akbar, M.F., Rana, H.U. and Khan, M.F., 2015b. Management of Bemicia tabaci Genn. on Solanum melongena L. through environmental friendly bio insecticides. Int. J. Biol. Biotechnol., 12: 393-399.
Amit, Y. and Raghuraman, M., 2014. Bioefficacy of certain newer insecticides against fruit and shoot borer, Leucinodes orbonalis (Guen.), white fly, Bemisia tabaci (Genn.), and jassid, Amrasca devastans Distant in brinjal. Ecoscan, 5(Spl. Issue): 85-89.
Anonymous, 2016. Agricu1tural statistics of Pakistan. Ministry of Food, Agriculture and Livestock, Food, Agriculture and Livestock Division, Economic Wing, Islamabad, pp. 27-28.
Asif, M.U., Muhammad, R., Akber, W. and Tofique, M., 2017. Relative efficacy of some insecticides against the sucking insect pest complex of cotton. Nucleus, 53: 140-146.
Chas, H.F. and Tilton, E.W., 1955. Tests with acaricides against the brown wheat mite. J. econ. Ent., 48: 157-161. https://doi.org/10.1093/jee/48.2.157
Cloyd-Raymond, A. and Bethke, J.A., 2011. Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manage. Sci., 67: 3-9. https://doi.org/10.1002/ps.2015
Collonier, C.I., Fock, V., Kasyhap, G.L., Rotino, M.C., Daunay, Y., Lian, I.K., Mariska, M.V., Rajam, A., Servaes, G. and Sihachakr, D., 2001. Application of biotechnology in eggplant. Pl. Cell Tissue Organ Cult., 65: 91-107. https://doi.org/10.1023/A:1010674425536
EGGNET, 2005. Management, conservation, and utilization of genetic resources of eggplants. Eggplant Genetic Resources Network. Available at: http://www.bgard.science,ru.nl/eggnet/eggnet01.html (accessed on 8 Sep, 2018).
FAOSTAT, 2017. Food and Agriculture Organization Statistical Database. Available at http://www.fao.org/faostat/en/#data/QC (retrieved January, 01, 2017).
Fauziah, I., Fairuz, K., Saiful, M., Jamaludin, M., Che Salmah, M.R. and Jusoff, K., 2009. Population ecology of whitefly, Bemisia tabaci, (Homoptera: Aleyrodidae) on brinjal. J. agric. Sci., 1: 27-35.
Gopal, D. and Islam, T., 2014. Relative efficacy of some newer insecticides on the mortality of jassid and white fly in brinjal. Int. J. Res. biol. Sci., 4: 89-93.
Handa, S.K., Agnihotri, N.P. and Kulshrestha, G., 1999. Pesticides residues, significance management and analysis, research periodicals and book publishing home. Texas, USA, pp. 226.
Henderson, C.F. and Tilton, E.W., 1955. Tests with acaricides against the brown wheat mite. J. Econ. Entomol., 48: 157-161. https://doi. org/10.1093/jee/48.2.157
Irshad, M., Abbas, G., Amer, M., Khokhar, M.B., Ahmad, M., Zakria, M. and Khan, G.A., 2015. Efficacy of different pesticides for the control of cotton jassid under the changing arid envirnoment of Thal zone. Int. J. Adv. Res. biol. Sci., 2: 121-126.
Kadam, D.B., Kadam, D.R., Umate, S.M. and Lekurwale, R.S., 2014. Bioefficacy of newer neonicotenoids against sucking insect pests of Bt cotton. Int. J. Pl. Protec., 7: 415-419. https://doi.org/10.15740/HAS/IJPP/7.2/415-419
Singh, G. and Kaushik, S., 1990. Comparative efficiency of sampling techniques for jassid population estimation on okra. Indian J. Ecol., 17: 58-60.
Khan, M.R., Ghani, I.A., Ghaffar, A. and Tamkeen, A., 2011. Host plant selection and oviposition behaviour of whitefly Bemisia tabaci (Gennadius) in a mono and simulated polyculture crop habitat. Afri. J. Biotechnol., 10: 1467-1472.
Kodandaram, M.H., Rai, A.B. and Haldar, J., 2010. Novel insecticides for management of insect pest in vegetable crops. Vegetable Sci., 37: 109-123.
Maji, T.B., Goswami, T.N., Das, A.K., Purkait, P. and Mukhopadhyay, A.K., 2015. Evaluation of buprofezin 70 DF an insect growth regulator for eco-friendly management of jassid (Amrasca bigutulla Ishida) in okra, Abelmoschus esculentus (L.) Moench. J. appl. Nat. Sci., 7: 725-728. https://doi.org/10.31018/jans.v7i2.673
Masud, S.Z. and Hasan, N., 1992. Pesticide residues in foodstuffs in Pakistan-organochlorine, organophosphorus and pyrethroid insecticides in fruits and vegetables. Pak. J. scient. Indust. Res., 35: 499-499.
Nadeem, M.K., Nadeem, S., Hasnain, M., Ahmed, S. and Ashfaq, M., 2011. Comparative efficacy of some insecticides against cotton whitefly, Bemisia tabaci (Genn.) (Homoptera: Aleyrodidae) under natural field conditions. Nucleus, 2: 159-162.
Norhelina, L., Sajap, A.S., Mansour, S.A. and Idris, A.B., 2013. Infectivity of five Metarhizium anisopliae (Deuteromycota: Hyphomycetales) strains on whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infesting brinjal, Solanum melongena (Solanaceae). Acad. J. Ent., 6: 127-132.
Omprakash, S. and Raju, S.V.S., 2014. A brief review on abundance and management of major insect pests of brinjal (Solanum Melongena L.). Int. J. appl. Biol. Pharm. Tech., 5: 228-238.
Pachundkar, N.N., Borad, P.K. and Patil, P.A., 2013. Evaluation of various synthetic insecticide against sucking insect pests of cluster bean. Int. J. Sci. Res. Publ., 3: 1-6.
Patnaik, M., Bhattacharya, D.K., Kar, N.B., Das, N.K., Saha, A.K. and Bindroo, B.B., 2011. Potential efficacy of new pesticides for the control of mulberry whitefly and its impact on silkworm rearing. J. Pl. Prot. Sci., 3: 57-60.
Peter, H.M., Yang, R.Y., Tsou, S.C.S., Ledesma, D., Engle, L. and Lee, T.C., 2006. Diversity in eggplant (Solanum melongena L.) for superoxide scavenging activity, total phenolics, and ascorbic acid. J. Fd. Com. Anal., 19: 594-600. https://doi.org/10.1016/j.jfca.2006.03.001
Ramalakshmi, V., Rao, G.M.V. and Madhumathi, T., 2012. Bioefficacy of novel insecticides against cotton leafhopper, Amrasca devastans. Annls. Pl. Protec. Sci., 20: 280-282.
Shaikh, A.A., Bhut, J.B. and Variya, M.V., 2014. Effectiveness of different insecticides against sucking pests in brinjal. Int. J. Pl. Protec., 7: 339-344. https://doi.org/10.15740/HAS/IJPP/7.2/339-344
Shivanna, B.K., Naik, B.G., Nagaraja, R., Basavaraja, M.K., Swamy C.M.K. and Karegowda, C., 2011. Bio efficacy of new insecticides against sucking insect pests of transgenic cotton. Int. J. Sci. Nat., 2: 79-83.
Vijay, B. and Ilyas, M., 2017. Evaluation of new insecticides against sucking pests of Bt cotton. Int. J. Pl. Anim. environ. Sci., 7: 66-72.
Wei, Z. and Liu, W., 1999. Kinetics and mechanism of the hydrolysis of imidacloprid. Pestic. Sci., 55: 482-485. https://doi.org/10.1002/(SICI)1096-9063(199904)55:4<482::AID-PS932>3.0.CO;2-3
To share on other social networks, click on any share button. What are these?










