Correlation Between Serum Level of Lymphokine (Interleukin-3) and Some Hematological Parameters in Polycystic Ovarian Syndrome Suffering Females from Sargodha Division, Pakistan
Correlation Between Serum Level of Lymphokine (Interleukin-3) and Some Hematological Parameters in Polycystic Ovarian Syndrome Suffering Females from Sargodha Division, Pakistan
Sajida Batool1, Mubeen Akhtar1, Shafaat Yar Khan1,2*
1Department of Zoology, University of Sargodha, Sargodha Pakistan
2Department of Zoology, Government College University Lahore Pakistan
Abstract | Polycystic ovarian syndrome (PCOS) is among the common endocrine disorders in childbearing aged females. This study is designed to compare a few anthropometric, hematological, and biochemical parameters in PCOS patients with that of normal females including correlation between interleukin-3 and blood cell count. Patients with PCOS and normal healthy females (n=60) of same age group were included in this study following Rotterdam ESHRE/ASRM criteria. The parameters evaluated were; white and red blood cells and platelets figure, lymphocytes, neutrophils, monocytes and eosinophils percentage and serum level of luteinizing hormone, follicle stimulating hormone and interleukin-3 in both groups. Two tailed, independent t-test and Pearson co-relation was applied to analyze hematological and biochemical parameters while anthropometric parameters like weight and height were compared as percentages. Results of current study showed that body mass index was significantly (P<0.05) elevated in PCOS patients when compared with BMI of control females. Number of successful pregnancies were noticed to be significantly (P<0.001) increased in control females. Percentage of infertile, hirsute and acne patients was higher in PCOS group, while serum interleukin-3 level was significantly (P<0.05) lower in PCOS patients than healthy participants. WBC count (P<0.01) and neutrophils % age (P<0.05) was significantly higher in PCOS group while eosinophils % age was statistically (P<0.01) decreased in PCOS patient in comparison to control subjects. RBCs and platelets count, lymphocytes and monocytes % age and neutrophils to lymphocytes ratio did not show any significant difference in control and deceased females. The mean LH/FSH ratio was significantly (P<0.05) elevated in PCOS patients than control. In female subjects of control group, IL-3 had significant positive correlation with WBC count (r=0.360, P<0.01) and significant negative correlation with Lymphocytes % age (r=-0.481, P<0.001) while in PCOS females no such correlation was observed. In conclusion, the increased body mass index, high neutrophils % age and WBCs count are closely associated to PCOS and may be responsible for inflammation in PCOS patients with decreased serum level of IL-3.
Novelty Statement | IL3 is well recognized as colony stimulating factor, which inhibits cell death and enhance the persistence of mega-karyocytes, macrophages and mast cell specifically in stress condition. This research is first contribution to find correlation of IL3 with hematological aspects in polycystic females.
Article History
Received: April 27, 2022
Revised: October 05, 2023
Accepted: October 28, 2023
Published: November 30, 2023
Authors’ Contributions
MA collected research sample, conducted the experiment, and wrote up the manuscript. SB designed, supervised the study, and write up. SYK guided and assisted in execution of experiments and analysis of data.
Keywords
Polycystic Ovarian Syndrom, IL-3, Hematology, Sargodha, Pakistan
Copyright 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding author: Shafaat Yar Khan
To cite this article: Batool, S., Akhtar, M., Khan, S.Y., 2023. Correlation between serum level of lymphokine (interleukin-3) and some hematological parameters in polycystic ovarian syndrome suffering females from Sargodha division, Pakistan. Punjab Univ. J. Zool., 38(2): 171-179.
Introduction
One of the common endocrine disorders is polycystic ovarian syndrome (PCOS) with worldwide prevalence of 4-12% in childbearing aged females (Kelestimur et al., 2006). It occurs when an ovarian follicle failed to mature enough to release egg (Petti and Secor, 2023). It is considered as the ruling cause of infertility in females (Jakubowski, 2005). As it contributes up to 56% in infertility cases (Khalid et al., 2022; Kanwal et al., 2022). Multiple reasons including genetic makeup, metabolic and environmental factors are accountable for PCOS. Stress, sedentary lifestyle, eating of canned food and toxicants contaminated environment are also chargeable to PCOS (Abinaya et al., 2019). Other malfunctioning associated with this disorder are puberty in childhood, obesity, irregular menstruation, acne, and hirsutism. PCOS females are reported to suffer from early adulthood infertility; cardiovascular diseases in middle stages of life, type II diabetes and in late age endometrial cancer like malignancies occur (Lentscher et al., 2021; Bloom et al., 2006; Norman et al., 2004). Almost 50–70% of PCOS females are reported with clinical signs of insulin resistance with reduced capacity of insulin to normalize glucose metabolism (Wang et al., 2021).
PCOS was firstly described by Stein and Levinthal (1935), but it is considered that genetic traits of this disorder are as ancient as 50,000 years ago (Azziz, 2016). Rosenfield (1999), criteria for the diagnosis of PCOS was established by NIH (National Institutes of Health)/NICHD (National Institutes of Child Health and Human Development) in a conference in 1990 that includes two features oligo-ovalution/ anovulation and biochemical/ clinical hyperandrogenism.
In 2003, Rotterdam ESHRE (European society of human reproduction and embryology)/ASRM (American society for reproductive medicine) elaborated the definition of PCOS as combination of oligomenorrhea or amenorrhea, polycystic ovaries (PCO) and endocrine signs of hyperandrogenemia are the main characteristics of PCOS. The patient must have any two features from above three. Ultrasound diagnostic criteria for PCO include 12 or more cysts per ovary having cyst size 2-9 mm diameter and volume of ovaries >10 ml in suspected female (Rotterdam and Group, 2004).
In 2006, the Androgen Excess Society modified the diagnostic criteria, which emphasize that hyperandrogenism must be an essential diagnostic element along either oligomenorrhoea/amenorrhea or polycystic ovaries (Azziz et al., 2006).
In early 90’s, LH/FSH ratio was considered as the main diagnostic feature for PCOS (Banaszewska et al., 2003) because an increased LH to FSH ratio was commonly found in PCOS patients (Taylor, 2006). LH/FSH ratio varies with physiological condition of female. PCOS females, almost 55–75% showed higher LH to FSH ratio. An increased level of LH and reduced FSH production in follicular phase of menstrual cycle is the cause of this elevation. GnRH stimulates excessive LH production in such women (Kalro et al., 2001). Criterion presented by Rotterdam in 2003 is considered most acceptable nowadays too (Sharquie et al., 2007).
Obese females are more susceptible to PCOS. Having a BMI greater than or equal to 30 kg/m2 is termed as obesity (Weir and Jan, 2022). Globally 38-88% females with PCOS are either obese or overweight. PCOS females have close association with hyperandrogenism that influence deposition of fats in adipose tissue and cause obesity in affected females (Barber et al., 2006). In PCOS hirsutism is common and visually can be diagnosed (Escobar et al., 2011). Excessive hair growth on the side of the face, chin and upper lip are included in hirsutism. Hairs on chest also have been observed in individuals with severe conditions of PCOS (Martin et al., 2008). Acne is common in PCOS patients and is related to dis-functioning of the pilosebaceous follicle (Tan and Bhate, 2015). Acne and excess of androgens are common features in patients of polycystic ovaries, Cushing syndrome and congenital adrenal hyperplasia (Cibula et al., 2000). Presence of acne in women with PCOS is 12–14%. Its prevalence may vary according to ethnicity as it is reported highest in South Asian women and lowest in Pacific areas. Comedones which are skin-colored small bumps often found on face due to accumulation of epithelial cell debris and sebum is major cause of acne. The bacterium Propionibacterium helps in colonization of acnes. In severe condition, inflammation occurs in comedones that give rise to papules and nodules on face (Deplewski and Rosenfield, 2000).
Cytokines are protein in nature and many of them are glycosylated. These small extracellular proteins perform function of signaling and have molecular size less than 80 kDa (Chung and Barnes, 1999). Every nucleated cell type can produce cytokines in the body. Cytokines perform regulatory functions in hematopoietic cells. In various other types of cells, they provide protection and help in repairing procedures (Thomson and Lotze, 2003). Pro inflammatory cytokines, lymphokines, inhibitory cytokines, growth factors and chemokine are the major groups of cytokines. T-lymphocytes also produce cytokines that are called lymphokines. Cytokines IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-15, IL-16 and IL-17 are included in the category of lymphokines (Mehta and Mahajan, 2006).
Interluekin-3 is a small chain polypeptide with α-helical configuration made up of 154 amino acids, and molecular weight 20-26 kDa. Mast cells, natural killer (NK) cells and activated T cells are the chief source of IL-3 (McNiece, 1997). IL3 have main function to regulate hematopoiesis. In animal models of human osteoarthritis and rheumatoid arthritis, it also prevents bone damage as well as cartilage damage (Kour et al., 2016). This lymphokine (IL-3) is a multi-potential hematopoietic growth factor. It induces proliferation and differentiation in pluripotent stem cells, progenitor myeloid stem cells, erythroid and megakaryocytic lineages (Eder et al., 1997; Mangi and Newland, 1999). Hematopoiesis starts during embryonic development and continue throughout adulthood to flourish and replenish the blood cells (Jagannathan-Bogdan and Zon, 2013).
Aims and objectives
This study is planned to evaluate comparison of following anthropometric, hematological, and biochemical parameters in PCOS patients with that of control subjects in Sargodha division:
- BMI, fertility ratio, childbearing ability, prevalence of abortions, acne, and hirsutism in PCOS patients and control females.
- Difference in blood cell count in PCOS and control participants.
- Hormonal status (FSH and LH) in the serum of both groups.
- Serum interleukin-3 level in PCOS patients and control group and to find correlation between IL-3 and blood cells count in both groups.
Materials and Methods
The experimental procedure of study was sanctioned by Institutional committee under Reference No. SU/Acad/129. Data was collected with the prior consent of participants either PCOS patients or as control. Data was based on:
- Performa/questionnaire that was designed to collect information about clinical and anthropometric variables and
- Blood sample to analyze different biochemical and hematological parameters.
In this case-control study, 120 women (n= 60) participated in two groups: PCOS group and Control group (healthy females).
PCOS suffering females were selected from different areas of Sargodha division. Healthy subjects were recruited from same localities. All the samples of both groups were collected from same age group ranging from 20-40 years old females.
A diagnostic criterion for PCOS patients was irregular menstrual cycle and cyst in ovaries as described in Rotterdam ESHRE/ASRM criteria (Rotterdam and Group, 2004). Only married females were included in this research. Females having any chronic disease like immunopathy, cardiovascular disorder, thyroid dysfunction, diabetes, cancer were not included in study group. Healthy subjects that did not have any disease history were included as control females.
Parameters mentioned in questionnaire were participant’s name, age, body mass index (BMI), fertility status, presence of polycystic ovary, acne, hirsutism, abortions, and record of successful pregnancies. BMI is the ratio of the weight of the body in kilograms to the square of its height in meters. Data was categorized in four groups according to BMI having categories underweight (<18.5 Kg/m2), normal (18.6-24.9 Kg/m2), overweight (25-29.9 Kg/m2) and obese (>30 Kg/m2).
Biochemical and hematological parameters were analyzed by taking 5 ml venous blood sample from all participants. 1ml blood was separated in Ethylene diamine tetraacetic acid (EDTA)-tube for complete blood cell count (CBC). CBC was done by Sysmex Kx-21 Hematology analyzer. 50µl blood was used in hematology analyzer. While, serum was obtained by clotting blood for 15 minutes at room temperature and centrifuging at 2500 rpm for 15 minutes. Serum was used for analysis of IL-3 and hormonal levels including LH and FSH by ELISA (Enzyme-Linked Immunosorbent Assay). IL-3 serum level was measured by using Kit of Bioassay Technology Laboratory, England (Catalogue number E0093Hu, sensitivity 1.02 pg/ml, intra-assay precision CV<8% and inter assay precision CV<10%). While, serum level of LH was assessed by the help of kit from Calbiotech (Catalog No LH231F, sensitivity 0.12mlU/ml, intra-assay and inter-assay precision CV% were (6.18-10.58) and (8.13-11.57), respectively). Similarly, serum FSH was evaluated by kit of CALBIOTECH Company (Catalog number FS232F, sensitivity 0.353mlU/ml, intra-assay and inter-assay precision CV% (6.3-6.7%) and (6.8-6.2%), respectively).
Statistics
The statistical analysis was performed using IBM statistical package for social sciences (SPSS) version 25. Changes among continuous variables between the two groups were analysed using the independent sample, two-tailed t-test.
Infertility ratio, prevalence of hirsutism and acne were calculated by using percentage formula.
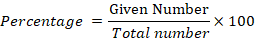
Correlation between IL-3 and blood cell count was measured by Pearson Correlation analysis.
Results and Discussion
Statistically, significant (P<0.05) increase in mean body mass index of PCOS patients was noticed as compared to control females as presented in Figure 1. Percentage of prevalence revealed that number of overweight and obese females was higher in PCOS patients as compared to control group (Table 1).
Table 1: Prevalence (%age) of females in different categories of body mass index in control and PCOS groups.
|
BMI (Kg/m2) |
Control (%) |
PCOS (%) |
|
Underweight (<18.5) |
3.4 |
1.6 |
|
Normal (18.6-24.9) |
86.2 |
51.6 |
|
Overweight (25-29.9) |
3.4 |
28.3 |
|
Obese (>30) |
6.8 |
18.3 |
Statistically (P<0.001) reduced number of successful pregnancies was noticed in PCOS females as compared to that of control females (Figure 2A). This indicated that presence of PCOS was altering the childbearing potential of patients. Data analysis of prevalence of abortion showed that there is no significant difference among control and PCOS participants in this parameter (Figure 2B).
High percentage of females was facing infertility (55%), hirsutism (40%), and acne (38.33%) issues in PCOS group as compared to that of control females (Table 2).
Table 2: Percentage of infertile, hirsute and acne patients among control and PCOS groups.
|
Parameters |
Control (%) |
PCOS (%) |
|
Infertility |
17.24 |
55 |
|
Hirsutism |
6.8 |
40 |
|
Acne |
3.44 |
38.33 |
Statistical analysis by two-tailed T-Test revealed that serum level of IL-3 was significantly (P<0.05) lower in PCOS patients as compared to control females (Figure 3).
Mean±SD values for various blood cells count and blood cell percentage in control and PCOS participants are presented in Table 3. Data analysis by independent sample, two-tailed T-Test showed that WBC count was significantly (P<0.001) higher in PCOS patients as compared to age-matched control females. Statistically non-significant difference in red blood cell and platelets count was found among control and PCOS bearing females. T-test showed that neutrophils % age was significantly higher (P<0.05) in PCOS subjects as compared to that of control females. Percentage of lymphocytes and monocytes was not statistically different in both groups. Non-significant neutrophils to lymphocytes ratio (NLR) was observed among both studied group. Significantly (P<0.01) higher eosinophils percentage was found in control females as compared to PCOS patients.
Table 3: Comparison of mean blood cells count and blood cell percentage in control and PCOS females.
|
Parameter (Blood cell count) |
Control |
PCOS |
P-value |
|
WBC (109/L) |
7.88±0.98 |
8.74±1.34 |
0.001*** |
|
RBC (1012/L) |
4.57±0.41 |
4.70±0.37 |
0.154 |
|
Platelets (109/L) |
268.34±40.71 |
284.01±51.54 |
0.124 |
|
Lymphocyte % |
35.04±4.72 |
36.82±6.86 |
0.157 |
|
Neutrophil % |
53.58±8.75 |
58.08±9.83 |
0.033* |
|
NLR |
1.56±0.40 |
1.66±0.51 |
0.236 |
|
Monocyte % |
5.62±2.65 |
4.83±2.06 |
0.167 |
|
Eosinophil % |
4.00±1.75 |
2.81±1.17 |
0.002** |
Values are expressed as Mean ± SD and comparison is made by two-tailed T-Test. P<0.05*, **P<0.01, ***P<0.001.
Table 4: Correlation between mean serum level of IL-3 and blood cell count in PCOS and healthy subjects.
|
Parameters |
Control |
PCOS |
||
|
R |
P |
R |
P |
|
|
WBC (109/L) |
0.360 |
0.005** |
0.111 |
0.339 |
|
RBC (1012/L) |
0.108 |
0.410 |
-0.035 |
0.788 |
|
Platelets (109/L) |
0.101 |
0.441 |
-0.198 |
0.724 |
|
Lymphocyte % |
-0.481 |
0.000*** |
0.220 |
0.129 |
|
Monocyte % |
-0.170 |
0.194 |
-0.029 |
0.827 |
|
Neutrophil % |
0.171 |
0.191 |
0.220 |
0.091 |
|
Eosinophil % |
0.144 |
0.247 |
-0.123 |
0.349 |
Data represented as Probability (P) and Pearson correlation (R) values and comparison is made by Pearson correlation analysis. **P<0.01, ***P<0.001.
In control subjects, IL-3 had significant positive correlation with WBC count (r=0.360, P<0.01) and highly significant negative correlation with lymphocytes percentage (r=-0.481, P<0.001) while in PCOS females no such correlation was observed (Table 4).
Statistical comparison of serum LH and FSH level showed noticeable (P<0.05) decrease in FSH concentration in serum of PCOS patients as compared to control, while LH serum level was not affected by the disease (Figure 4A). LH/FSH ratio was statistically (P<0.05) higher in PCOS patients than that of healthy females (Figure 4B), and likewise LH/FSH ratio in fertile and infertile PCOS participants was higher (P<0.01) in comparison to fertile and infertile control subjects (Figure 4C).
PCOS is among most communal endocrine disorders. Current research included study of a cytokine IL-3, some hormonal and hematological parameters in the association of PCOS.
In this study, mean BMI value was statistically higher in PCOS participants than healthy females. Among PCOS patients, 28.3% were overweight (BMI>25Kg/m2) and 18.3% obese (BMI>30Kg/m2). In PCOS patients, sex steroid metabolism was disturbed due to suppression of hepatic production of sex hormone binding globulin modulated by hyperinsulinemia, which results in increase in the level of androgens and adiposity in these females (Bremer and Miller, 2008; La Marca et al., 2000; Lim et al., 2012). Excess of androgens promote visceral adiposition (Sam, 2007). Increased adiposity due to altered production of androgens might be the cause of overweight and obese subjects in PCOS group in the current study. Number of fertile females was significantly (P<0.001) more in healthy group as compared to PCOS, as 55% PCOS females of this study were infertile. According to Homburg (2004) anovulation is major cause in ~75% PCOS women suffering from infertility.
Clark et al. (2014) reported that 13% of women with PCOS have idiopathic hirsutism and poorer metabolic profile compared to controls. In current study, 40% PCOS patients were suffering from hirsutism as they had facial hairs on upper lips, chin and chest region. Hirsutism is an important clinical symptom of hyperandrogenism (Escobar et al., 2011). Hyperandrogenism might be the cause of hirsutism in PCOS patients as compared to age matched control females in this study.
In this study, there were 38.8% PCOS females having moderate acne. In another research, 36 % mild, 40% moderate and 24% severe acne was observed in PCOS patients. Maximum patients with PCOS showed moderate acne (Iftikhar and Choudhry, 2019). They proved least association of androgens with acne severity, but androgenic hormones were elevated in the acne patients than control females. Hormonal imbalance due to PCOS might directly or indirectly generate psychological issues for females like hirsutism.
In reproductive field, IL-3 is one of the least studied cytokines. Recently a study has been conducted on polymorphism in gene regulating Interleukin-3 in association of miscarriage in fresh in-vitro fertilized (IVF) oocytes. Wu et al. (2019) reported that IL-3 rs40401 C/T polymorphisms had higher abortion rate in IVF patients.
In this study, serum interleukin-3 level was significantly (P<0.05) lower in PCOS patients as compared to control females. PCOS seem to effect the secretion of this lymphokine in patients. IL-3 showed significant positive correlation with WBC count (r=0.360, P<0.01) and highly significant negative correlation with Lymphocyte % age (r=-0.481, P<0.001) in control females while in PCOS females no such correlation was observed in current research. In studies of late 1990’s artificial administration of dose of IL-3 to patients resulted in increased number of leukocytes (mainly eosinophils, neutrophils, and lymphocytes), reticulocytes and platelets (Eder et al., 1997; Mangi and Newland, 1999).
In this study, white blood cell number was statistically increased (P<0.01) in PCOS females than healthy females. Papalou et al. (2015) also reported that WBC number was higher (P<0.01) in the PCOS patients than control females of similar age. Increased WBC count is a good inflammatory marker and PCOS patients showed increased leucocytes count (Orio et al., 2005). Higher WBC count could be regarded as an inflammatory marker in PCOS participants as also observed in this study.
RBC and platelets count was non-significantly elevated in the PCOS patients than control group. Similarly, non-significant difference in RBC and platelets count among control and PCOS patients was also reported in other studies (Dereli et al., 2003; Yilmaz et al., 2016).
In present study, non-significant increase in lymphocytes %age in blood was found in PCOS females as compared to control participants. In a study conducted by Yilmaz et al. (2016) significant (P<0.05) increase in lymphocytes % age in PCOS patients than control group was noticed. PCOS patients have significant elevation in macrophages and lymphocytes count when compared to control (Xiong et al., 2011). Increased level of lymphocytes in PCOS patients can be a step toward chronic inflammation.
In this study neutrophils % age was significantly elevated in PCOS (P<0.05) patients as compared to control females. Evaluated neutrophils count in both lean and obese PCOS patients was observed in other studies (Kurt et al., 2014; Yilmaz et al., 2016). PCOS stimulated hyperneutrophilia might be a risk factor to general health of patients inducing metabolic disorders.
In current study, NLR was not significantly different in PCOS patients as compared to control females. Contrarily, Kurt et al. (2014) reported increased NLR in patients with PCOS females as compared to the control group. Furthermore, they suggested that increased inflammation might be associated with increased NLR and possibly it is one of the main patho-physiological mechanisms in PCOS.
In this study monocytes % age was non-significantly different in the control and PCOS group. DincgezCakmak et al. (2018) also reported non-significant (P= 0.852) level of monocytes count in PCOS and control group. This study showed highly significant (P<0.01) eosinophils % age in the PCOS patients as compared to control females. Variable reports are available in this context as according to a study conducted in Turkey, it was reported that eosinophils count remained non-significantly different (P= 0.670) in PCOS patients and control group females (Yilmaz et al., 2016), while in another study conducted in China significantly increased eosinophils count in PCOS females (P<0.01) was found in PCOS females as compared to control group females (Xiong et al., 2011). Different results in these studies might be due to variations in study areas and racial population under observation.
In present study, blood samples were collected from participants to check serum hormonal ratio (LH/FSH). Significant increase in LH/FSH ratio was observed in PCOS females as compared to control females. LH/FSH ratio was significantly high (P<0.05) in fertile and infertile PCOS patients. Results of this study are constant with another Pakistani study held by Haider et al. (2011). Their sampling days were 6th to 30th day of menstrual cycle. Among Pakistani population, their results depicted 71% of PCOS females having elevated LH/FSH ratio. Similarly, Iwasa et al. (2009) conducted a study in which they assessed LH/FSH ratio in both initial and final follicular phase of PCOS bearing females.
Conclusions and Recommendations
This study concludes that altered level of gonadotropin especially FSH might contribute in severity of disease generating psychological and health issues like hirsutism, acne and infertility. High neutrophils % age and WBC count showed close association to disease and might be playing inductive role in inflammation and metabolic complications in PCOS patients. Low level of serum interleukin-3 in PCOS females showed its close alliance with PCOS.
Acknowledgment
We acknowledge all the females who participated in the study. We also acknowledge the clinicians, paramedics and their institutions who facilitated during the study.
Conflict of interest
The authors have declared no conflict of interest.
References
Abinaya, S., Siva, D., Sabitha, R. and Achiraman, S., 2019. An overview of hyperandrogenism in pcos and the prospective underlying factors.
Azziz, R., 2016. Introduction: Determinants of polycystic ovary syndrome. Fertil. Steril., 106: 4-5.
Azziz, R., Carmina, E., Dewailly, D., Diamanti-Kandarakis, E., Escobar-Morreale, H.F., Futterweit, W. and Taylor, A.E., 2006. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J. Clin. Endocrinol. Metab.,91: 4237-4245. https://doi.org/10.1210/jc.2006-0178
Banaszewska, B., Spaczynski, R., Pelesz, M. and Pawelczyk, L., 2003. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo-and hyperinsulinemia. Rocz. Akad. Med. Bialymst., 48: 131-134.
Barber, T., McCarthy, M., Wass, J. and Franks, S., 2006. Obesity and polycystic ovary syndrome. Clin. Endocrinol., 65: 137-145. https://doi.org/10.1111/j.1365-2265.2006.02587.x
Bloom, M.S., Schisterman, E.F. and Hediger, M.L., 2006. Selecting controls is not selecting “normals”: design and analysis issues for studying the etiology of polycystic ovary syndrome. Fertil. Steril., 86: 1-12. https://doi.org/10.1016/j.fertnstert.2005.09.066
Bremer, A.A. and Miller, W.L., 2008. The serine phosphorylation hypothesis of polycystic ovary syndrome: A unifying mechanism for hyperandrogenemia and insulin resistance. Fertil. Steril., 89: 1039-1048. https://doi.org/10.1016/j.fertnstert.2008.02.091
Chung, K. and Barnes, P., 1999. Cytokines in asthma. Thorax, 54: 825-857. https://doi.org/10.1136/thx.54.9.825
Cibula, D., Hill, M., Vohradnikova, O., Kuzel, D., Fanta, M. and Zivny, J., 2000. The role of androgens in determining acne severity in adult women. Br. J. Dermatol., 143: 399-404. https://doi.org/10.1046/j.1365-2133.2000.03669.x
Clark, N.M., Podolski, A.J., Brooks, E.D., Chizen, D.R., Pierson, R.A., Lehotay, D.C. and Lujan, M.E., 2014. Prevalence of polycystic ovary syndrome phenotypes using updated criteria for polycystic ovarian morphology: an assessment of over 100 consecutive women self-reporting features of polycystic ovary syndrome. Reprod. Sci., 21: 1034-1043. https://doi.org/10.1177/1933719114522525
Deplewski, D. and Rosenfield, R.L., 2000. Role of hormones in pilosebaceous unit development. Endocr. Rev., 21: 363-392. https://doi.org/10.1210/edrv.21.4.0404
Dereli, D., Ozgen, G., Buyukkececi, F., Guney, E. and Yilmaz, C., 2003. Platelet dysfunction in lean women with polycystic ovary syndrome and association with insulin sensitivity. J. Clin. Endocrinol. Metab., 88: 2263-2268. https://doi.org/10.1210/jc.2002-021391
DincgezCakmak, B., Dundar, B., KetenciGencer, F., Aydin, B.B. and Yildiz, D.E., 2018. TWEAK and monocyte to HDL ratio as a predictor of metabolic syndrome in patients with polycystic ovary syndrome. Gynecol. Endocrinol., 35: 66-71. https://doi.org/10.1080/09513590.2018.1490401
Eder, M., Geissler, G. and Ganser, A., 1997. IL-3 in the clinic. Stem Cell., 15: 327-333. https://doi.org/10.1002/stem.150327
Escobar-Morreale, H., Carmina, E., Dewailly, D., Gambineri, A., Kelestimur, F., Moghetti, P. Witchel, S., 2011. Epidemiology, diagnosis and management of hirsutism: A consensus statement by the androgen excess and polycystic ovary syndrome society. Hum. Reprod. Update, 18: 146-170. https://doi.org/10.1093/humupd/dmr042
Haider, S., Manan, N., Khan, A. and Qureshi, M.A.Q., 2011. Prevalence of elevated luteinizing hormone (LH)/follicle stimulating hormone (FSH) ratio in polycystic ovary syndrome (PCOS) women among local population. J. Dow Univ. Hlth. Sci., 5: 17-20.
Homburg, R., 2004. Management of infertility and prevention of ovarian hyperstimulation in women with polycystic ovary syndrome. Best Pract. Res. Clin. Obst. Gynaecol., 18: 773-788. https://doi.org/10.1016/j.bpobgyn.2004.05.006
Iftikhar, U. and Choudhry, N., 2019. Serum levels of androgens in acne and their role in acne severity. Pak. J. Med. Sci., 35: 146. https://doi.org/10.12669/pjms.35.1.131
Iwasa, T., Matsuzaki, T., Murakami, M., Shimizu, F., Kuwahara, A., Yasui, T. and Irahara, M., 2009. Reproducibility of luteinizing hormone hypersecretion in different phases of the menstrual cycle in polycystic ovary syndrome. J. Obstet. Gynaecol. Res., 35: 514-519. https://doi.org/10.1111/j.1447-0756.2008.00998.x
Jagannathan-Bogdan, M. and Zon, L.I., 2013. Hematopoiesis. Development, 140: 2463-2467. https://doi.org/10.1242/dev.083147
Jakubowski, L., 2005. Genetic aspects of plycystic ovary syndrome. Endokrynol. Pol., 56: 285-291.
Kalro, B.N., Loucks, T.L. and Berga, S.L., 2001. Neuromodulation in polycystic ovary syndrome. Obstet. Gynecol. Clin. N. Am., 28: 35-62. https://doi.org/10.1016/S0889-8545(05)70184-4
Kanwal, H. I., Shahid, M. and Bacha, R., 2022. Sonographic evaluation of various causes of female infertility: A literature review. J. Diagn. Med. Sonogr., 38: 155-159. https://doi.org/10.1177/87564793211052023
Kelestimur, F., Unluhizarci, K., Baybuga, H., Atmaca, H., Bayram, F. and Şahin, Y., 2006. Prevalence of polycystic ovarian changes and polycystic ovary syndrome in premenopausal women with treated type 2 diabetes mellitus. Fertil. Steril., 86: 405-410. https://doi.org/10.1016/j.fertnstert.2006.01.019
Khalid, N.H.M., Ahmed, I.A.M. and Ahmed, S.A.F., 2022. Evaluation of causes of female infertility using ultrasonography in Najran, Saudi Arabia. Afr. J. Reprod. Hlth., 26: 90-95.
Kour, S., Garimella, M.G., Shiroor, D.A., Mhaske, S.T., Joshi, S.R., Singh, K. and Chattopadhyay, N., 2016. IL-3 decreases cartilage degeneration by downregulating matrix metalloproteinases and reduces joint destruction in osteoarthritic mice. J. Immunol., 196: 5024-5035. https://doi.org/10.4049/jimmunol.1500907
Kurt, R.K., Okyay, A.G., Hakverdi, A.U., Gungoren, A., Dolapcioglu, K.S., Karateke, A. and Dogan, M.O., 2014. The effect of obesity on inflammatory markers in patients with PCOS: A BMI-matched case–control study. Arch. Gynecol. Obstet., 290: 315-319. https://doi.org/10.1007/s00404-014-3199-3
La Marca, A., Egbe, T.O., Morgante, G., Paglia, T., Ciani, A. and De Leo, V., 2000. Metformin treatment reduces ovarian cytochrome P-450c17α response to human chorionic gonadotrophin in women with insulin resistance-related polycystic ovary syndrome. Hum. Reprod., 15: 21-23. https://doi.org/10.1093/humrep/15.1.21
Lentscher, J. A., Slocum, B. and Torrealday, S., 2021. Polycystic ovarian syndrome and fertility. Clin. Obst. Gynecol., 64: 65-75. https://doi.org/10.1097/GRF.0000000000000595
Lim, S.S., Davies, M., Norman, R.J. and Moran, L., 2012. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update, 18: 618-637. https://doi.org/10.1093/humupd/dms030
Mangi, M.H. and Newland, A.C., 1999. Interleukin-3 in hematology and oncology: Current state of knowledge and future directions. Cytokines, Cell. Mol. Ther., 5: 87-95.
Martin, K.A., Chang, R.J., Ehrmann, D.A., Ibanez, L., Lobo, R.A., Rosenfield, R.L. and Swiglo, B.A., 2008. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab., 93: 1105-1120. https://doi.org/10.1210/jc.2007-2437
McNiece, I., 1997. Interleukin-3 and the colony-stimulating factors. Cytokines in health and disease, 2nd ed. Marcel Dekker, Ann Arbor, Mich, pp. 41-53.
Mehta, A.A. and Mahajan, S., 2006. Role of cytokines in pathophysiology of asthma. Iran. J. Pharmacol. Ther., 5: 1-0.
Norman, R., Wu, R. and Stankiewicz, M., 2004. Polycystic ovary syndrome. Med. J. Aust., 180: 132-137. https://doi.org/10.5694/j.1326-5377.2004.tb05838.x
Orio, F., Palomba, S., Cascella, T., Di Biase, S., Manguso, F., Tauchmanovà, L. and Russo, T., 2005. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J. Clin. Endocrinol. Metab., 90: 2-5. https://doi.org/10.1210/jc.2004-0628
Papalou, O., Livadas, S., Karachalios, A., Tolia, N., Kokkoris, P., Tripolitakis, K. and Diamanti-Kandarakis, E., 2015. White blood cells levels and PCOS: direct and indirect relationship with obesity and insulin resistance, but not with hyperandogenemia. Hormones, 14: 91-100. https://doi.org/10.14310/horm.2002.1563
Petti, Y.M. and Secor, R.M., 2023. Polycystic ovarian syndrome. Advanced health assessment of women: Skills, procedures, and management, pp. 363. https://doi.org/10.1891/9780826179630.0027
Rosenfield, R.L., 1999. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol. Metab. Clin. N. Am., 28: 265-293. https://doi.org/10.1016/S0889-8529(05)70070-0
Rotterdam, E. and Group, A.S.P.C.W., 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril., 81: 19. https://doi.org/10.1016/j.fertnstert.2003.10.004
Sam, S., 2007. Obesity and polycystic ovary syndrome. Obes. Manage., 3: 69-73. https://doi.org/10.1089/obe.2007.0019
Sharquie, K.E., Al-Bayatti, A.A., Al-Ajeel, A.I., Al-Bahar, A.J. and Al-Nuaimy, A.A., 2007. Free testosterone, luteinizing hormone/follicle stimulating hormone ratio and pelvic sonography in relation to skin manifestations in patients with polycystic ovary syndrome. Saudi Med. J., 28: 1039.
Stein, I.F., 1935. Amenorrhea associated with bilateral polycystic ovaries. Am. J. Obstet. Gynecol., 29: 181-191. https://doi.org/10.1016/S0002-9378(15)30642-6
Tan, J. and Bhate, K., 2015. A global perspective on the epidemiology of acne. Br. J. Dermatol., 172: 3-12. https://doi.org/10.1111/bjd.13462
Taylor, A.E., 2006. Gonadotropin dysfunction in women with polycystic ovary syndrome. Fertil. Steril., 86: S12. https://doi.org/10.1016/j.fertnstert.2006.05.001
Thomson, A.W. and Lotze, M.T., 2003. The cytokine handbook. Gulf Professional Publishing. Vol. 1.
Wang, H., Zhang, Y., Fang, X., Kwak-Kim, J. and Wu, L., 2021. Insulin resistance adversely affect IVF outcomes in lean women without pcos. Front. Endocrinol., 12: 734638. https://doi.org/10.3389/fendo.2021.734638
Weir, C.B. and Jan, A., 2022. BMI classification percentile and cut off points. StatPearls. Treasure Island (FL).
Wu, C.H., Lee, T.H., Yang, S.F., Tsao, H.M., Chang, Y.J., Chou, C.H. and Lee, M.S., 2019. Interleukin-3 polymorphism is associated with miscarriage of fresh in vitro fertilization cycles. Int. J. Environ. Res. Publ. Hlth.,16: 995. https://doi.org/10.3390/ijerph16060995
Xiong, Y.l., Liang, X.Y., Yang, X., Li, Y. and Wei, L.N., 2011. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol., 159: 148-150. https://doi.org/10.1016/j.ejogrb.2011.07.012
Yilmaz, M., Duran, C. and Basaran, M., 2016. The mean platelet volume and neutrophil to lymphocyte ratio in obese and lean patients with polycystic ovary syndrome. J. Endocrinol. Invest., 39: 45-53. https://doi.org/10.1007/s40618-015-0335-2
To share on other social networks, click on any share button. What are these?







