Detection of Insecticide Residues in Honey of Apis dorsata F. from Southern Punjab, Pakistan
Detection of Insecticide Residues in Honey of Apis dorsata F. from Southern Punjab, Pakistan
Muhammad Aslam Farooqi1,*, Mansoor-ul-Hasan2, Sohail Akhtar1,
Muhammad Arshad2, Muhammad Naveed Aslam3 and Muhammad Rafay4
1Department of Entomology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Pakistan
2Department of Entomology, Faculty of Agriculture, University of Agriculture, Faisalabad, Pakistan
3Plant Pathology Section, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
4Department of Forestry, Range and Wildlife Management, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
ABSTRACT
A simple and fast analytical method using High Performance Liquid Chromatography with ultraviolet detection (HPLC-UV) was used to determine residues of commonly used agricultural insecticides (imidacloprid, thiametoxam, profenofos, endosulfan, spinosad and deltamethrin) in multi-floral raw honey of Apis dorsata F. collected from the cotton belt area of Punjab, Pakistan. The residues of these insecticides were extracted using ethyl acetate. Honey samples were spiked at 0.1 and 0.01 mg/kg. The mean recoveries of these insecticides in the spiked samples were 74-92% with relative standard deviation less than 20%. The residues of imidacloprid, endosulfan and deltamethrin were detected with a range of 0.005-0.055 mg/kg while other insecticides were not detected in any samples. The results obtained, point the urgent need to establish reliable monitoring programs in honey.
Article Information
Received 23 October 2016
Revised 11 January 2017
Accepted 25 March 2017
Available online 07 September 2017
Authors’ Contribution
MAF performed the experiments and wrote the article, MH and MA conceived and designed the study and SA, MNA and MA help in data analysis
Key words
Insecticides, Residues, Raw honey, HPLC-UV.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1761.1766
* Corresponding author: aslam_farooqi1770@yahoo.com
0030-9923/2017/0005-1761 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Application of insecticides on different crops has resulted in serious threat to non-target organisms and human health (Rambabu and Roa, 1994). Honeybees are good bio-indicators of different contaminants in environment due to close contact with insecticides and other chemical substances during their foraging on different flowering plants (Bozena, 2002). Insecticides are transferred to the honey by the bees, as they collect pollens and nectars from different plants, where these have been applied (Devillers and Pham-Delegue, 2002; Bogdanov, 2006). The widespread use of insecticides has created a need for their monitoring in different food products (Hussain et al., 2001).
Insecticide residues determination in bee products is necessary to monitor contamination for safe consumer health (Fernandez et al., 2002; Rissato et al., 2007), to assess the potential risk of this product to consumers. Maximum residue limit (MRLs) of different insecticides in different foodstuffs including honey has been set by European Union Commission (EU) for the safety of consumers and to regulate international trade (European Commission, 2006). The main objective of this study was to determine the residues of commonly used insecticides in honey samples of giant honeybee (A. dorsata) which were being used in cotton belt area of Punjab, Pakistan against different insect pests of field crops.
Materials and Methods
Chemical standards, reagents and solvents
Certified analytical standards of profenofos, deltamethrin, imidacloprid thiamethoxam, spinosad and endosulfan (Table I) were purchased from their respective manufacturing companies. Their purity was > 98% except spinosad which was 88.4% pure. Distal water was obtained with the help of glass-distilled and further purified with the help of a Millipore Milli-Q water purifier. HPLC grade acetonitrile, ethyl acetate, Sodium chloride and anhydrous sodium sulphate. C18-bonded silica (50 μm), florisil (60-100 mesh) was purchased from Merck limited.
| Trade name | Common name | Chemical group | Toxicity |
|
Confidor @200 SL |
Imidacloprid | Nicotinoid | II/WHO |
| Actara @25 WG | Thiamethaxam | Nicotinoid | III/WHO |
| Decis @2.5 EC | Deltamethrin | Pyrethyroid | II/WHO |
| Curacron @500 EC | Profenofos | Organophosphate | II/WHO |
|
Thiodan @35 EC |
Endosulfan | Organochlorin | II/WHO |
| Tracer @240 SC | Spinosad | Bio Insecticide |
IV/WHO |
WHO (2000) and Fishel (2010).
Collection of honey Samples
For residues determination of insecticides, 16 samples of multi-floral raw honey of Apis dorsata F. were collected from four Districts including Multan, D.G. Khan, Layyah and Bahawalpur during the harvesting season of honey in 2013-14. Four samples were collected from each District, and then stored in dark at 10 °C until analysis.
Standard stock solutions
The insecticides standard stock solutions were individually prepared in acetone by dissolving 20 mg in 25 ml of solvent and were stored in a freezer at -18 °C. The stock standard solutions were used up to 3 months. Suitable concentrations of working standards were prepared from the stock solutions by dilution using acetonitrile, immediately prior to sample preparation.
Residues extraction
Rissato et al. (2004) method was used with some modifications for insecticide residues extraction with ethyl acetate.
Cleaning of samples
The samples were cleaned up by adding 0.5 g silica gel, 1g anhydrous sodium sulphate, 5g mixture of activated carbon and silica gel or florisil. The samples were passed through a chromatographic column and then the filtered eluate was received, the collected extracts were dried under a gentle stream of pure nitrogen (N2). Then 1 ml of ethyl acetate was added to this eluate and was submitted to analysis by High Performance Liquid Chromatography equipped with ultraviolet (HPLC-UV).
Method validation
For validation, the parameters accuracy, precision, linearity and limits of detection (LOD) and limit of quantification (LOQ) were considered. The accuracy of the method was determined by recovery tests; using samples spiked at two different levels of 0.1 and 0.01 mg/kg with known concentration of the pure insecticides standard solution of each type and extraction and cleaning were performed as described earlier. The concentration of each insecticide in the final extracts was calculated. Recovery studies were performed to examine the efficacy of extraction and cleaned up. These recovery tests of insecticides were necessary to meet the requirements of the European Commission 31, which indicate that a method can be considered accurate and precise when the accuracy of data is between 70 and 110%, with Relative Standard Deviations (RSDs) not higher than 20%. Linearity was determined by different known concentrations which were prepared by diluting the stock solutions.
Chromatographic separation parameters
The HPLC-UV system was used for determination of insecticide residues (Alyaseri et al., 2012; Rao et al., 2012) in honey, consisting Shimadzu High Performance Liquid Chromatography with LC-20AT pump and SPD- 20A and was interfaced with LC solution software, was equipped with a reversed Phase C-18 analytical column of 250 mm×4.6 mm and particle size 5.0 μm (Phenomenex). Column temperature was maintained at 30 °C. The injected sample volume was 20 μL. Mobile Phases A and B were acetonitrile and Milli -Q water (75:25(v/v)). The flow- rate used was kept at 1.2 mL/min. The detector wavelength was 230 nm. The external standard method was used for these analyses.
Identification and calculation
The compounds were identified by comparing the retention times of the samples peaks with that of the standard peaks and the amount of residues (mg/kg) was recorded in the integrator chart. The amount of residues in mg/kg is calculated as follows (Kumari et al., 2003):
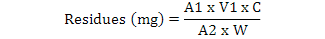
Where, A1 is area of the sample in chromatogram, A2 is area of the standard in chromatogram, V1 is total volume of the sample in mL, C is concentration of analytical standard in ug/mL, W is weight of the sample in g and RF is recovery/response factor.
| Insecticides |
H.S. Rec. % (RSD) |
L.S. Rec. % (RSD) |
| Imidacloprid |
92 (5.6) |
86 (5.8) |
| Thiamethaxam |
81 (6.1) |
82 (3.6) |
| Deltamethrin |
84 (11.3) |
77 (6.1) |
| Profenofos |
89 (6.7) |
83 (4.8) |
| Endosulfan |
81 (7.6) |
77 (3.8) |
| Spinosad |
78 (7.6) |
74 (4.3) |
Values are converted into percentage and RSD stands for relative standard deviation (if RSD is less than 20% the method is considered well for residue determination).
Results and Discussion
Recoveries of insecticide residues
The recovery tests of different insecticides were performed by the analysis of honey samples through method validation because validation is a prerequisite of any reliable chromatographic analysis (Levison et al., 1995). In this method linearity of calibration curve, sensitivity and selectivity of the solute detection, reproducibility, instrument precision, detection limit, quantification limit, and recovery of insecticide residues were performed (Lee et al., 1995). Honey samples were spiked with two different concentrations of pure insecticide standards at 0.1 mg and 0.01 mg/kg. The range of recoveries of these insecticides in the spiked samples was 74-92% and the range of Relative Standard Deviation was 3.8-11.3%. These values were quite satisfactory and met the requirements of the European Commission Regulations (2000) (Table II).
Contamination results of real honey samples
Table III shows residues of imidacloprid, thiamethoxam, deltamethrin, endosulfan, profenofos and spinosad in 10 out of 16 honey samples analyzed. Maximum contamination was observed with imidacloprid detected in 50% of samples, followed by endosulfan and deltamethrin, detected in 37.5% and 31.2% of samples, respectively. The residues concentrations of imidacloprid endosulfan and deltamethrin, ranged from 0.012-0.055, 0.007-0.026 and 0.01-0.023 mg/kg, respectively. Imidacloprid and endosulfan were detected at the highest levels of 0.55 and 0.26 mg/kg, respectively and levels exceeded maximum residue limits (MRLs) from the Districts of Bahawalpur and Multan based on European Commission (EC) Regulation in honey.
Table III.- Insecticide residues detected in honey (mg/kg) from Southern Punjab.
| S. No | Sample Code |
Imidacloprid |
Thiametoxam |
Spinosad |
Deltamethrin |
Endosulfan |
Profe nofos |
| 1 |
DGK1 |
0.012±0.001 |
Nd |
Nd |
Nd |
0.007±0.004 |
Nd |
| 2 |
DGK2 |
0.033±0.001 |
Nd |
Nd |
Nd |
Nd |
Nd |
| 3 |
DGK3 |
Nd |
Nd |
Nd |
Nd |
Nd |
Nd |
| 4 |
DGK4 |
Nd |
Nd |
Nd |
0.023±0.01 |
Nd |
Nd |
| 5 |
MTN1 |
Nd |
Nd |
Nd |
Nd |
Nd |
Nd |
| 6 |
MTN2 |
0.018±0.015 |
Nd |
Nd |
0.019±0.01 |
0.008±0.06 |
Nd |
| 7 |
MTN3 |
Nd |
Nd |
Nd |
Nd |
Nd |
Nd |
| 8 |
MTN4 |
0.013±0.01 |
Nd |
Nd |
0.015±0.01 |
0.026*±0.01 |
Nd |
| 9 |
BWP1 |
0.026±0.022 |
Nd |
Nd |
Nd |
0.007±0.002 |
Nd |
| 10 |
BWP2 |
0.055*±0.03 |
Nd |
Nd |
0.01±0.003 |
0.007±0.002 |
Nd |
| 11 |
BWP3 |
Nd |
Nd |
Nd |
Nd |
Nd |
Nd |
| 12 |
BWP4 |
Nd |
Nd |
Nd |
Nd |
Nd |
Nd |
| 13 |
LYH1 |
0.011±0.002 |
Nd |
Nd |
Nd |
Nd |
Nd |
| 14 |
LYH2 |
0.043±0.024 |
Nd |
Nd |
Nd |
0.009±0.021 |
Nd |
| 15 |
LYH3 |
Nd |
Nd |
Nd |
0.031±0.012 |
Nd |
Nd |
| 16 |
LYH4 |
Nd |
Nd |
Nd |
Nd |
Nd |
Nd |
Values are expressed as means ± standard deviation and are means of triplicate samples.*, residue exceeding MRL; Nd, not detected; DGK, D.G. Khan; MTN, Multan; BWP, Bahawalpur; LYH, Layyah.
The MRLs of pesticides, legally permitted in honey, has been established by different countries usually about acaricides. However, the European Union (EU) legislation has regulated the MRLs for different insecticides in honey (European Commission, 2006) which are given in Table IV.
Table IV.- Maximum residues limits of insecticides in mg/kg, studied in honey.
| Insecticides | MRL | Insecticides | MRL |
| Imidacloprid | 0.05 | Profenofos | 0.01 |
| Thiamethaxam | 0.01 | Endosulfan | 0.01 |
| Deltamethrin | 0.03 | Spinosad | 0.05 |
Several researchers have studied the insecticides contamination in various honey samples collected from different regions of the world previously for monitoring of insecticide residues. In a previous study, 50 pesticide residues detected in 26 honeys from Jordan (Al-Rifai and Akeel, 1997). In a study conducted in Indian, 55% samples of honey were contaminated with residues of different class of insecticides (Anju et al., 1997) with a detection range of 0.01-9 mg/kg and from Spain, 38% contamination of honey was observed with different pesticides residues (Garcia et al., 1995). Previous investigations (Blasco et al., 2003) detected organochlorines, organophosphates and carbamates insecticides residues in honey from Portugal and Spain, with residues ranging 0.03-4.31 mg/kg. Contamination of honey with different pesticides has been also reported from France (Chauzat and Faucon, 2007) and Switzerland (Bogdanov et al., 2003).
It is difficult to compare our results with those of other monitoring programs from other countries, because the range of pesticides detected varies from many parts of the world (Blasco et al., 2004; Jimenez et al., 2002; Martel and Zeggane, 2002). The quantification limits of different insecticide residues detected in honey ranged from 0.1 to 0.6 mg/kg for organochlorin pesticides and from 5.0-25.0 ug/kg for organophosphates in a study from Aragon, Spain (Herrera et al., 2005). Residues of endosulfan, organophosphates and pyrethroids were detected in honey and were above MRLs previously reported from Brazil (Rissato et al., 2007). In developed countries, gas chromatography (GC) and liquid chromatography (LC) equipped with mass spectrometry (MS) are recently being used for the multi-residues detection and quantifications of pesticides in various monitoring programs of honey samples due to their potential to detect and quantify various pesticides relatively in a short time period with minimum extraction of samples. The current results of the contamination of honey with different insecticides residues from Pakistan, indicates that their presence is an alarming situation and there is an urgent need to monitor their contents in honey for consumers safety.
Conclusion
The results obtained from the present investigations, clearly indicated that there is a significant contamination of insecticides in multi-floral honey produced from Apis dorsata F. in Southern Punjab. The maximum contamination was observed with imidacloprid.
Acknowledgements
The author pays his special thanks to Prof. Dr. Anjum Suhail (Late) Ex-Chairman, Department of Entomology, University of Agriculture, Faisalabad due to his kind help for the completion of this research work and to Higher Education Commission (HEC) of Pakistan for providing financial support under Indigenous PhD Fellowship.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Al-Rifai, J. and Akeel, N., 1997. Determination of pesticide residues in imported and locally produced honey in Jordan. J. Apic. Res., 36: 155-161. https://doi.org/10.1080/00218839.1997.11100943
Anju, R., Beena, K., Gahlawat, S., Sihag, R. and Kathpal, T., 1997. Multiresidue analysis of market honey samples for pesticidal contaminaton. J. Pestic. Res., 9: 226-230.
Bempah, C.K. and Donkor, A.K., 2011. Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ. Monit. Asses, 175: 551-561. https://doi.org/10.1007/s10661-010-1550-0
Blasco, C., Fernandez, M., Pena, A., Lino, C., Silveira, M.I. and Font, G., 2003. Assessment of pesticide residues in honey samples from Portugal and Spain. J. Agric. Fd. Chem., 51: 8132–8138. https://doi.org/10.1021/jf034870m
Blasco, C., Lino, C., Picó, Y., Pena, A., Font, G. and Silveira, M.I., 2004. Determination of organochlorine pesticide residues in honey from the central zone of Portugal and the Valencian community of Spain. J. Chromatogr. A, 1049: 155-160. https://doi.org/10.1016/j.chroma.2004.07.049
Bogdanov, S., 2006. Contaminants of bee products. Apidologie, 37: 1-18. https://doi.org/10.1051/apido:2005043
Bozena, M., 2002. Simple method for the determination of trace levels of pesticides in honeybees using matrix solid phase dispersion and gas chromatography. Plant protection institute in Poznan. J. Chromatogr. A, 982: 267-273. https://doi.org/10.1016/S0021-9673(02)01505-4
Chauzat, M.P. and Faucon, J.P., 2007. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Manage. Sci., 63: 1100-1106. https://doi.org/10.1002/ps.1451
European Commission, 2000. Quality control procedures for pesticide residues analysis, SANCO/3103/2000. Guidelines for residues monitoring in the European Union.
European Commission, 2006. Commission amending Regulation (EC) No 396/2005 (updated on 08/10/2013) of The European Parliament and of the Council to establish Annex I listing the food and feed products to which maximum levels for pesticide residues apply. Official Journal of European Union.
Fernandez, M., Pico, Y., and Manes, J., 2002. Analytical methods for pesticide residue determination in bee products. J. Fd. Protect., 65: 1502–1511. https://doi.org/10.4315/0362-028X-65.9.1502
Fidente, P., Seccia, S., Vanni, F. and Morrica, P., 2005. Analysis of nicotinoid insecticides residues in honey by solid matrix partition clean-up and liquid chromatography electrospray mass spectrometry. J. Chromatogr. A, 1094: 175-178. https://doi.org/10.1016/j.chroma.2005.09.012
Fishel, F.M., 2010. The EPA conventional reduced risk pesticide program. UF/IFAS EDIS Document PI-224. Available at: http://edis.ifas.ufl.edu/pi224.
Garcia, M.A., Fernandez, M.I. and Melgar, M.J., 1995. Contamination of honey with organophosphorus pesticides. Bull. environ. Contam. Toxicol., 54: 825-832. https://doi.org/10.1007/BF00197965
Hamilton, D.D., Ambrus, A., Dieterle, R., Felsot, A., Harris, C., Petersen, B., Racke, K., Wong, S.S., Gonzalez, R. and Tanaka, K., 2004. Pesticide residues in food-acute dietary exposure. Pest Manage. Sci., 60: 311-339. https://doi.org/10.1002/ps.865
Herrera, A., Arquillue, C.P., Conchello, P., Bayarri, S., Lazaro, R., Yague, C. and Arino, A., 2005. Determination of pesticides and PCBs in honey by solid phase extraction cleanup followed by gas chromatography with electron-capture and nitrogen phosphorus detection. Analyt. Bioanalyt. Chem., 381: 695-701. https://doi.org/10.1007/s00216-004-2924-3
Hussain, S., Masud, T. and Ahad, K., 2002. Determination of pesticides residues in selected varieties of mango. Pak. J. Nutr., 1: 41-47. https://doi.org/10.3923/pjn.2002.41.42
Jimenez, J., Bernal, J., Toribio, J.L., Nozal, L. and Martin, M.T., 2002. Capillary gas chromatography with mass spectrometric and atomic emission detection for characterization and monitoring chlordimeform degradation in honey. J. Chromatogr. A, 946: 247–253. https://doi.org/10.1016/S0021-9673(01)01588-6
Lee, J.W., Naidong, W., Johnson, T., Dzerk, A., Miyabashi, T. and Motohashi, M., 1995. Development and validation of column switching high performance liquid chromatographic method for the determination of a potent all receptor antagonist TCV-116 and its metabolites in human serum and urine. J. Chromatogr. B, 670: 287-98. https://doi.org/10.1016/0378-4347(95)00146-8
Levison, P.R., Badger, S.E., Jones, R.M.H., Toome, T.W., Streater, M., Pathirana, N.D. and Wheeler, S., 1995. Validation studies in the regeneration of ion exchange cellulose. J. Chromatogr. A, 702: 59-68. https://doi.org/10.1016/0021-9673(94)00991-H
Martel, A.C. and Zeggane, S., 2002. Determination of acaricides in honey by high -performance liquid chromatography with photodiode array detection. J. Chromatogr. A, 954: 173–180. https://doi.org/10.1016/S0021-9673(02)00126-7
Rambabu, P.J. and Roa, M.B., 1994. Effect of organochlorine and three organophosphate pesticides on glucose, glycogen, lipid and protein contents in tissues of freshwater snails Bellamya dissilis (Muller). Environ. Contam. Toxicol., 53: 142-148.
Rissato, S.R., Galhiane, M.S., Knoll, F.N. and Apon, B.M., 2004. Supercritical fluid extraction for pesticide multi-residue analysis in honey: determination by gas chromatography with electron-capture and mass spectrometry detection. J. Chromatogr. A, 1048: 153–159. https://doi.org/10.1016/S0021-9673(04)01213-0
Rissato, S.R., Galhiane, M.S., Almeida, M.V., Gerenutti, M. and Apon, B.M., 2007. Multi-residue determination of pesticides in honey samples by gas chromatography–mass spectrometry and application in environmental contamination. Fd. Chem., 101: 1719-1726. https://doi.org/10.1016/j.foodchem.2005.10.034
WHO, 2000. Recommended classification of pesticides by hazard and guide lines to classification Geneva, World Health Organization (document reference WHO/PCS/01.4)










