Drought Tolerance Screening in Thirty Common Wheat (Triticum aestivum L.) Genotypes
Drought Tolerance Screening in Thirty Common Wheat (Triticum aestivum L.) Genotypes
Ajmalud Din1*, Munir Ahmad2, Fahad Masoud Watto2, Sheraz Ahmed1, Imtiaz Ali1 and Muhammad Kausar Nawaz Shah2
1Department of Plant Breeding and Genetics, The University of Agriculture, Peshawar, 25130, Khyber Pakhtunkhwa, Pakistan; 2Department of Plant Breeding and Genetics, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, 46300, Pakistan.
Abstract | Drought stress is a serious threat limiting global wheat production. Adaptability of wheat genotypes to drought stress is an important objective in wheat breeding. This study was carried out to understand the effect of drought stress on growth and development, and to identify drought tolerant wheat genotypes. Thirty wheat genotypes of diverse genetic makeup were assessed in transparent polythene bags (6 × 27 inches), and filled them with sterile, homogenized mixture of loam, sand and compost soil. Two factorial Completely Randomized Design was followed with three replications and two treatments. The experiment was conducted during 2015-16 in the experimental site of the Department of Plant Breeding and Genetics, PMAS Arid Agriculture University Rawalpindi, Pakistan. Data were recorded on drought-related physiological traits viz. proline content, osmotic adjustment, excised leaf water retention (ELWR), relative water content (RWC), cell membrane stability (CMS), chlorophyll content and canopy temperature. Mean squares for all traits revealed significant differences among genotypes under moisture stress and normal environmental conditions. High variations were recorded for cell membrane stability, relative water content and osmotic adjustment which indicated the suitability of these traits as selection criterion for drought tolerance. Significant association was observed between chlorophyll and proline content. Osmotic adjustment was significantly correlated with relative water content and cell membrane stability. According to obtained results, genotypes NARC-09, AUR-10, SA-75, Mexipak and AUR-09 were identified as drought-tolerant, hence can serve as a good source in wheat breeding programs to develop high yielding varieties for arid, drought and rainfed areas.
Received | October 29, 2018; Accepted | December 01, 2019; Published | February 01, 2020
*Correspondence | Ajmalud Din, Department of Plant Breeding and Genetics, The University of Agriculture, Peshawar, 25130, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Din, A., M. Ahmad, F.M. Watto, S. Ahmed, I. Ali and M.K.N. Shah. 2020. Drought tolerance screening in thirty common wheat (Triticum aestivum L.) genotypes. Sarhad Journal of Agriculture, 36(1): 168-177.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.1.168.177
Keywords | Wheat, Drought tolerance, Proline, Osmotic adjustment, Canopy temperature
Introduction
The common wheat (Triticum aestivum L.) has been cultivated since the beginning of civilization. It is the topmost staple food for more than 1/3rd of the world’s population (Abd-El-Haleem et al., 2009). Being staple food, it is used as the main food commodity by more than 200 million people in Pakistan. Wheat grains provide 20% of world’s diet calories. It contains 70% carbohydrates, 22% crude fibers, 12% proteins, 12% water, 2% fat and 1.8% minerals gram-1 (Mahpara, 2008).
Water is very essential for smooth running of various metabolic activities inside plants. In absence of water, the growth and development of plants decreases substantially. Water deficiency in arid and semi-arid areas is a serious concern for sustainable agriculture all over the world (Sharafi et al., 2011). In semiarid areas, drought causes significant reduction in wheat production (Ali et al., 2011). Statistical data represent that drought stress affects more than 99 million hectares in developing countries and 60 million hectares in developed countries (Rajaram, 2000). In Pakistan, an area of 15 million hectares is affected by water deficiency (Mujtaba and Alam, 2002). Water deficiency can reduce grain yield from 17 to 70% in wheat (Nouri-Ganbalani et al., 2009). Besides appropriate irrigation system, about 1.2 billion hectares cultivated area of the world is rainfed and have very low yield potential.
Drought is a big challenge for plant breeders around the world. To feed the increasing population of the world under such environmental conditions, plant breeders need to develop cultivars which could sustain such stress conditions without significant yield loss (Edgerton, 2009). Water stress adversely affects the plant growth, development and slow down the process of optimal productivity (Farooq et al., 2011a; Ahmadizadeh, 2013). In drought conditions, plants usually respond in the form of stunted growth due to its adverse effects on different molecular, biochemical, physiological and morphological processes of the plant. Such changes are totally related to the growth stage, time and severity of environmental stresses (Cao et al., 2011). For screening and selection of tolerant genotypes to uphold productivity under water stress conditions, the understanding of physiological mechanisms is very essential (Zaharieva et al., 2001). For instance, it is reported that drought stress had severely minimized germination and development of seedling in wheat (Nezhadahmadi et al., 2013). Similarly, drought related physiological parameters were dramatically reduced under water stress conditions as compared to control (Maqbool et al., 2015).
Plants typically show a large number of physiological responses under water deficit conditions including relative water content (RWC) (Machado and Paulsen, 2001). Plant exposure to moisture stress lowers down the relative water content, leaf water potential and osmotic potential (Grover et al., 2004). Relative water content is a good indicator of drought tolerance in plants than leaf water potential (Merah, 2001). Different studies revealed that RWC is the best measure for classification and screening of water stress-tolerant genotypes (Lugojan and Ciulca, 2011; Farshadfar et al., 2012; Hasheminasab et al., 2012) as it is associated with high yield and other yield related components (Akram, 2011). Under abiotic stresses, wheat varieties with higher cell membrane stability (Blum et al., 2001) and chlorophyll content (Khakwani et al., 2012) tended to produce higher yield. The use of such physiological traits at seedling and vegetative stage for drought evaluation is an effective and economical way to identify tolerant genotypes at early stage (Rehman et al., 2016). Keeping in view the above narrated facts, the objectives of this research work were to; i) study the impact of drought stress on physiological traits of wheat genotypes, and ii) identify drought tolerant genotypes for arid, semiarid and rainfed areas of Pakistan.
Materials and Methods
Experiment layout and design
This study was carried out during 2015-16 at the experimental site of the Department of Plant Breeding and Genetics, PMAS Arid Agriculture University Rawalpindi. List of 30 common wheat genotypes is presented in Table 1. Experimental material was raised in transparent polythene bags in the shelter. Polythene bags (6 × 27 inches) were filled with homogenized mixture of loam, sand and compost. Eight seeds per polythene bag were sown to a depth of 2-3 cm and after emergence, seedling numbers per pot were reduced to four. The experiment comprised of two treatments (control and stress) with three replications. All polythene bags were watered equally with tape water. For stress treatment, water stress was imposed by holding water after tillering stage. Each bag (four seedlings) was considered as single replication. Hence in each treatment, there were 12 seedlings per genotype. The experiment was conducted following a completely randomized design. Data from three plants per polythene bag were recorded at vegetative stage for different physiological traits viz. estimation of proline, osmotic adjustment, relative water content, excised leaf water retention, cell membrane stability, canopy temperature and chlorophyll content as detailed below.
Chlorophyll Content (%)
Chlorophyll content was measured by chlorophyll meter Minolta SPAD 502. Chlorophyll meter was placed on flag leaf base, center and the tip and readings were noted. Three plants from each replication of both treatments were randomly selected and then averaged to note chlorophyll content for each treatment.
Table 1: List of 30 wheat genotypes studied.
| Sr. No. | Genotypes | Sr. No. | Genotypes |
| 1 | Pakistan-13 | 16 | Chakwal-97 |
| 2 | Shahkar-13 | 17 | Sariab-92 |
| 3 | Anmol-91 | 18 | Inqalab-91 |
| 4 | AARI-011 | 19 | Pirsabak-91 |
| 5 | Punjab-011 | 20 | AUR-10 |
| 6 | AAS-011 | 21 | Bahawalpur 2000 |
| 7 | Millat-011 | 22 | Bwp-97 |
| 8 | NARC-09 | 23 | Chakwal-86 |
| 9 | Chakwal-50 | 24 | Barani-83 |
| 10 | Pirsabak-08 | 25 | Sarhad-82 |
| 11 | Lasani-08 | 26 | Pak-81 |
| 12 | AUR-09 | 27 | SA-75 |
| 13 | Pirsabak-05 | 28 | Lyp-73 |
| 14 | Bhakkar-02 | 29 | Mexipak |
| 15 | Fakhre-Sarhad | 30 | LU-26 |
Relative water content (%)
Fresh leaves from each treatment were collected and weighed to record fresh weight (FW). Turgid weight (TW) was measured after placing it in distilled water for 4 hr. Thereafter, oven-dried the selected leaf segments at 72 °C for 48 h and weighed again to find out dried weight (DW). RWC was calculated using the formula given by Egert and Tevini (2002).
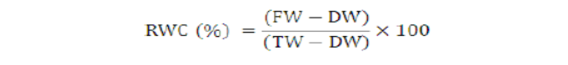
Osmotic adjustment (MPa)
Irrigation was given to treatments for saturation before sampling in the afternoon and then covered with a transparent plastic sheet. Flag leaves were put into Eppendorf tubes and placed the sample in deep freezer. Cell sap was extracted after thawing and centrifugation and then osmotic potential was recorded by using Osmometer. The osmotic potential was measured in mmol kg-1 and then converted to MPa (pressure unit) by the formula as used by Nobel (1991):
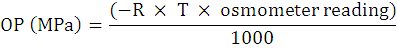
Where:
R= 0.008314 (gas constant); T= laboratory temperature. OA is measured by OA = OPnon-stressed – OPstressed
Cell membrane stability (%)
Fully expanded uppermost leaves were taken as leaf samples and cut into 1 cm pieces following Premachandra and Shimada, (1988). Leaf sample (0.5g) was cut into pieces and put in deionized water for 90 minutes. During this time, deionized water was changed three times at different time intervals. Two sets were made separately for control and stress. Control samples were kept in 15 ml deionized distilled water at 10 °C for 24 hours. Whereas, stress samples were kept into 15 ml 25% polyethylene glycol (PEG 6000) solution at 10 °C for 24 hours. Again, the samples were kept in deionized water for 90 minutes while changing deionized water at different time intervals. After that, both sets were kept in 15 ml deionized distilled water at 10 °C for 24 hours. Electrical conductivity (EC) of control (C1) and stress (T1) samples were measured by using EC meter. Then, autoclaved the leaf samples of both sets for 15 minutes at 121 °C. The second electrical conductivity of control (C2) and stress (T2) was measured. CMS value was estimated by using the following equation;
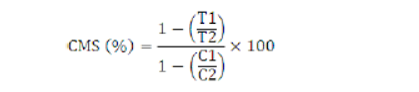
Canopy temperature (°C)
Canopy temperature was measured by using Infrared Thermometer (Model AG-42, Tela-temp Crop, Fullerton, CA.). One measurement per polythene bag was taken from nearly 50 cm above the canopy with an angle of 30° from the horizontal. Data presented for each treatment was the mean of three sets of measurements made pre-heading between 12:00 and 16:00 hours.
Excised leaf water retention (%)
Prior to anthesis, the second leaf was taken and weighed (FW), left for 4 hr at 25°C and reweighed in the wilted form (WW4h). ELWR was measured by using the following formula as used by Farshadfar et al. (2002).
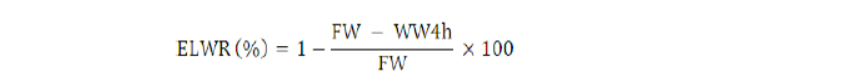
Proline concentration (µg/g)
Proline content was measured according to Bates et al. (1973) by using acid ninhydrin reagent. Fresh leaf samples (250 mg each) were homogenized in 5 ml of 3% aqueous sulfosalicylic acid and then centrifuged for 15 minutes at 12000 rpm by keeping the temperature 4 °C. About 2 ml supernatant was taken and mixed with the same amount of acid ninhydrin and acetic acid. Then, incubated the samples in test tubes at 100 °C for 1 hr. Terminate the reaction in the ice bath after incubation. Add 4 ml toluene to the product and shake gently for 15-20 seconds. Optical density was measured using spectrophotometer (Spekol 1300) at the wavelength of 520 nm against the blank containing toluene. A standard curve was made and the quantity of proline was quantified from standard curve. Proline content was determined by following the formula of Bates et al. (1973):
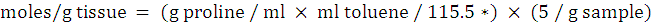
Where;
115.5 = molecular weight of proline.
Statistical analysis
Data collected for various physiological traits viz. proline content, osmotic adjustment, excised leaf water retention (ELWR), relative water content (RWC), cell membrane stability (CMS), chlorophyll content and canopy temperature were subjected to the analysis of variance (ANOVA) following Steel et al., (1997). Means of genotypes were separated using duncan’s multiple range test. Correlation coefficients were computed using statistical analysis system computer software (SAS, 2009).
Results and Discussion
Analysis of variance
Mean squares for various physiological traits of varieties, treatments and treatments × varieties are presented in Table 2. Results revealed that significant variability existed among varieties for all the studied traits and both the treatments expressed significant impacts on all characters studied. Interaction due to treatments by varieties (T×V) was also significant for all the studied traits. The significant results indicated that varieties’ tolerance level varied against drought stress.
Evaluation of varieties on the basis of their average performance
The mean performance of each trait for all varieties under both treatment conditions is graphically represented in Figure 1 to Figure 8. The maximum, minimum and mean values of all varieties for each studied trait under both environments are given in Table 3. Overall, chlorophyll content under normal condition was 41.6% while 45.3% under water stress conditions. This indicated that the chlorophyll content increased significantly under drought conditions which is similar to the results reported by Hennouni et al. (2012). Generally, higher chlorophyll content indicates less photoinhibition to the photosynthetic apparatus and increases drought resistance in plants. Mean values for chlorophyll content ranged between 35.47 and 47.73% in normal growing plants whereas, from 34.77 to 55.83% in water-stressed plants. Hence, results revealed that more variation existed for chlorophyll content under both normal and stress conditions. The minimum chlorophyll content was observed for variety Mexipak under both normal (35.47%) and stress (34.77%) conditions. Under normal condition, maximum chlorophyll content was exhibited by Shahkar-13 (47.73%) followed by Bahawalpur-2000 (46.93%) while Lasani-08 (55.83%) had the highest mean value for chlorophyll content followed by Pirsabak-05 (55.73%) in drought stress condition as showed in Figure 1.
Table 2: Mean Square eight physiological traits studied under normal and stress conditions.
| Variables | Varieties | Treatments | T×V | CV |
| Chlorophyll content (CC) | 73.61** | 1174.02** | 37.21** | 6.66 |
| Canopy Temperature (CT) | 4.62** | 50.138** | 6.855** | 6.09 |
| Proline Content (PC) | 0.0563** | 0.1332** | 0.0042** | 10.14 |
| Osmotic Potential (OP) | 1.435** | 5.000** | 2.368** | 3.37 |
| Relative Water Content (RWC) | 119.97** | 6869.32* | 109.93* | 9.85 |
| Excised Leaf Water Retention (ELWR) | 66.321** | 983.503** | 58.578* | 6.75 |
| Cell Membrane Stability (CMS) | 2545.65** | 9.35 | ||
| Osmotic Adjustment (OA) | 5.970* | 6.40 |
*: Significant; **: Highly Significant; ns: Non-Significant.
According to the results shown in Table 3, mean value of all varieties for canopy temperature under normal conditions was recorded as 24.31°C while under drought stress condition was recorded as 25°C. Data for canopy temperature ranged between 22.43 and 26.20°C under normal conditions whereas, under stress, it ranged between 21.90 and 28.10°C. According to Figure 2, maximum mean temperature was observed for Chakwal-50 (26.20°C) followed by AARI-011 (25.63°C) under normal conditions whereas under stress conditions, variety Anmol-91 (28.10°C) had maximum canopy temperature followed by AUR-09 (27.93°C). The minimum canopy temperature was observed for Bwp-97 (22.43°C) followed by Mexipak (22.67°C) under normal condition while Fakhre-Sarhad (21.90°C) and Barani-83 (22.33°C) in stress condition. Results revealed that varieties under non-stress conditions had high canopy temperature as compared to stress. This could be due to the fact that canopy temperature is related to the rate of transpiration in plants. Plants with low canopy temperatures show more transpiration, which generates cooling effect. Contrarily, plants having high canopy temperature indicate the shortage of water in soil as drought.
Table 3: Descriptive statistics of eight physiological traits in thirty wheat varieties under normal and stress conditions.
| Traits | Normal | Stress | ||||
| Max. | Min. | Mean | Max. | Min. | Mean | |
| CC | 47.73 | 35.47 | 41.6 | 55.83 | 34.77 | 45.3 |
| CT | 26.20 | 22.43 | 24.315 | 28.10 | 21.90 | 25 |
| PC | 0.479 | 0.133 | 0.306 | 0.493 | 0.154 | 0.3235 |
| OP | -0.00010 | -0.00016 | -0.00013 | -0.00014 | -0.00020 | -0.00017 |
| RWC | 95.922 | 78.396 | 87.159 | 88.830 | 60.316 | 74.573 |
| ELWR | 93.712 | 75.202 | 84.457 | 96.226 | 80.005 | 88.1155 |
| CMS | 149.745 | 33.025 | 91.385 | |||
| OA | 5.965 | 1.112 | 3.5385 | |||
CC: Chlorophyll Content; CT: Canopy Temperature; PC: Proline Concentration; OP: Osmotic Potential; RWC: Relative Water Content; ELWR: Excised Leaf Water Retention; CMS: Cell Membrane Stability and 0A: Osmotic Adjustment.
Under normal conditions, the mean performance of all varieties for proline content was 0.306 µg/g while 0.323 µg/g under drought condition (Table 3). These results indicated that proline concentration increased under stress conditions which is similar to the findings of Chorfi and Taibi (2011) and Hennouni et al. (2012). Mean values for proline content ranged from 0.133 to 0.479 µg/g under normal conditions while from 0.154 to 0.493 µg/g under drought conditions. Differences in means suggested a greater variation under both conditions. Minimum proline content was observed by AUR-09 (0.133 µg/g) followed by Chakwal-50 (0.143 µg/g) under normal while Millat-011 (0.154 µg/g) followed by Chakwal-50 (0.157 µg/g) under stress environment. Similarly, higher proline content was recorded for Pak-81 (0.479 µg/g) followed by Sarhad-82 (0.475 µg/g) under normal conditions. Pak-81 (0.493 µg/g) followed by Shahkar-13 (0.492 µg/g) possessed higher proline content under stress condition (Figure 3). Chorfi and Taibi (2011) also reported that varieties that had higher proline content were more likely to perform better under drought conditions, therefore, screening of germplasm for drought tolerance based on this parameter was very useful.
Overall, osmotic potential under normal condition was -0.00013 mmol kg-1, while -0.00017 mmol kg-1 under stress condition. Data for osmotic potential ranged between -0.00016 and -0.00010 mmol kg-1 under normal whereas -0.00020 and -0.00014 mmol kg-1 under water stress condition. According to Figure 4, maximum osmotic potential under stress environment was observed for Barani-83 (-0.00014 mmol kg-1) followed by Chakwal-86 and LU-26 (-0.00015 mmol kg-1) while Millat-011, Barani-83 and LU-26 (-0.00010 mmol kg-1) followed by AUR-09 (-0.00011 mmol kg-1) under normal. Similarly, Lasani-08, Bhakkar-02, Sariab-92 and Pirsabak-91 exhibited the minimum (-0.00016 mmol kg-1) osmotic potential under normal conditions whereas, Bhakkar-02 and Pirsabak-91 (-0.00020 mmol kg-1) had minimum osmotic pressure under stress condition. The degree of osmotic potential indicates the solute concentration of the cell. Due to osmoregulation, plants that could maintain high turgor pressure usually survives under drought conditions. These results were similar to the findings of Sayar et al. (2008), that efficient osmoregulation was the key phenomenon in drought tolerant varieties.
The mean performance of all varieties under normal condition for relative water content was 87.16 %, while 74.57% under stress condition. These findings are similar to those reported by Keshavars, et al. (2012) that under stress conditions plants had lower relative water content. The RWC ranged from 78.396 to 95.922% under normal condition, whereas from 60.316 to 88.83% under water stress conditions. These results indicated the existence of sufficient variation for this character under both conditions. Variety LU-26 had lowest mean value (78.396%) under normal, while SA-75 (60.32%) had lowest mean value for RWC under stress environment. Similarly, NARC-09 exhibited higher mean value (95.922%) for RWC followed by Bwp-97 (94.578%) under normal experiment, whereas AUR-10 (88.83%) possessed higher mean value under water stress conditions (Figure 5). This indicated that the varieties had more RWC under normal conditions than stress. Similar findings were also reported by Gunes et al. (2008). Hasheminasab et al. (2012) advocated the use of relative water content as a key selection measure to identify drought-tolerant varieties.
The mean value for excised leaf water retention (ELWR) under normal condition was 84.457% while under stress it was 88.115% (Table 3). Under normal condition, the mean ELWR ranged from 75.202 to 93.712% whereas from 80.005 to 96.226% under stress condition. These findings indicated differential performance of varieties for ELWR under both environmental conditions. Lower mean value for ELWR was recorded for variety Barani-83 (75.202%) under normal while AUR-10 had higher (80.005%) mean value for ELWR under stress condition. Higher mean values for ELWR were recorded for Sariab-92 (93.71%) followed by Pirsabak-91 (92.564%) under normal conditions, whereas Pirsabak-91 (96.23%) followed by Shahkar-13 (95.95%) had higher mean values under drought condition (Figure 6). Results revealed that under stress conditions excised leaves tended to lose less water than normal condition because of the scarcity of water in leaves. Generally, drought tolerant plants have more ELWR under stress conditions. Therefore, under stress conditions, higher excised leaf water retention has been proposed as a key water status indicator in drought tolerant plants (Gunes et al., 2008).
Mean data for cell membrane stability was 91.385% (Table 3). Mean values of CMS ranged from 33.025 to 149.745%. Th results indicated the substantial influence of drought stress on cell membrane stability which are in line with Razzaq et al. (2013). Overall, lowest cell membrane stability was observed for Chakwal-86 (33.025%) followed by NARC-09 (40.725%), whereas higher membrane stability was observed for SA-75 (149.745%) followed by Fakhre-Sarhad (136.36%) (Figure 7). The CMS indicates the normal functionality cellular machinery. Therefore, plant with higher CMS could perform reasonably better under drought conditions than those having lower CMS (Sairam et al., 2002).
Average osmotic adjustment of 30 wheat varieties was 3.538 MPa. Overall, mean values ranged from 1.112 to 5.965 MPa. Significant phenotypic variations for osmotic adjustment were observed among all the varieties. Similar results were reported by Moinuddin et al. (2005). Lower value for osmotic adjustment varieties was recorded for Sarhad-82 (1.112 MPa) followed by Chakwal-86 (1.434 MPa), whereas NARC-09 (5.965 MPa) followed by Millat-011 (5.872 MPa) had higher value as represented in Figure 8. It has been reported that varieties with higher osmotic adjustment had superior performance under drought stress conditions (Ming et al., 2012).
Correlation analysis among various physiological traits
Correlation coefficients under normal conditions: The coefficient of correlation is the measurement of the linear relationship between two variables. The Pearson correlation of six physiological parameters under control conditions is presented in Table 4. Chlorophyll content had positive correlation with proline content only which is supported by the findings of Khaket et al. (2014), whereas it had negative correlation with all other studied traits under normal condition. Cell membrane stability demonstrated positive correlation with canopy temperature, osmotic adjustment and proline content while expressed negative correlation with excised leaf water retention and relative water content. According to Farooq et al. (2011b), CMS is a major component of drought tolerance and its positive association with mentioned physiological traits indicated stability under moisture stress conditions. Canopy temperature expressed positive correlation with excised leaf water retention and relative water content while it showed negative correlation with osmotic adjustment and proline content. Excised leaf water retention demonstrated positive correlation only with canopy temperature and negative correlation with other five studied physiological traits. These results are in agreement with the findings of Geravandi et al. (2011). Osmotic adjustment represented positive correlation with relative water content while negative with proline content as described by Naeem et al. (2016). Proline content expressed negative correlation with relative water content. These findings are in accordance with earlier results of Geravandi et al. (2011) and Rehman et al. (2016). This indicates that genotypes with high CMS will have low RWC which make them impotent for water stress condition.
Table 4: Pearson correlation eight physiological traits under control conditions.
| CC | CMS | CT | ELWR | OA | OP | Proline | |
| CMS | -0.216 | ||||||
| CT | -0.088 | 0.016 | |||||
| ELWR | -0.093 | -0.002 | 0.087 | ||||
| OA | -0.293 | 0.072 | -0.122 | -0.142 | |||
| OP | 0.239 | 0.170 | -0.439 | -0.133 | -0.174 | ||
| Proline | 0.282 | 0.069 | -0.305 | -0.213 | -0.423 | 0.226 | |
| RWC | -0.117 | -0.081 | 0.031 | -0.120 | 0.131 | -0.354 | -0.089 |
Chlorophyll Content (CC); Cell Membrane Stability (CMS); Canopy Temperature (CT); Excised Leaf Water Retention (ELWR); Osmotic Adjustment (OA); Proline Concentration (PC); Relative Water Content (RWC).
Correlation coefficients under stress condition
In the stressed environment, chlorophyll content was positively correlated with canopy temperature, excised leaf water retention and proline content while it expressed negative correlation with cell membrane stability, osmotic adjustment and relative water content (Table 5). According to the findings of Shamsi (2010), drought-tolerant wheat cultivars had higher chlorophyll content under water stress conditions. Cell membrane stability demonstrated positive correlation with excised leaf water retention and osmotic adjustment as also observed by Naeem et al. (2016), while it expressed negative correlation with canopy temperature, proline content and relative water content. Under abiotic stresses, wheat varieties with higher cell membrane stability and chlorophyll content tended to produce higher yield as also reported by Blum et al. (2001). Canopy temperature expressed positive correlation with excised leaf water retention, osmotic adjustment and relative water content while negative with proline content. So, cooler canopy temperature could be used as a selection criterion for drought-tolerant genotypes as suggested by Olivares-Villegas et al. (2007). Excised leaf water retention demonstrated positive correlation with all studied physiological traits except proline content. Osmotic adjustment represented positive correlation with relative water content while negative with proline content. Proline content expressed negative correlation with all studied traits except chlorophyll content. Drought tolerance at a molecular level is associated with the capability to accumulate proline and high-water level conservation (Sultan et al., 2012).
Table 5: Pearson correlation eight physiological traits under stress conditions.
| CC | CMS | CT | ELWR | OA | OP | Proline | |
| CMS | -0.129 | ||||||
| CT | 0.058 | -0.209 | |||||
| ELWR | 0.056 | 0.017 | 0.093 | ||||
| OA | -0.074 | 0.072 | 0.112 | 0.162 | |||
| OP | 0.008 | 0.195 | -0.188 | -0.033 | 0.527 | ||
| Proline | 0.199 | -0.145 | -0.173 | -0.164 | -0.407 | -0.113 | |
| RWC | -0.037 | -0.120 | 0.073 | 0.029 | 0.354 | -0.081 | -0.051 |
Chlorophyll Content (CC); Cell Membrane Stability (CMS); Canopy Temperature (CT); Excised Leaf Water Retention (ELWR); Osmotic Adjustment (OA); Proline Concentration (PC); Relative Water Content (RWC).
Conclusions and Recommendations
Based on higher OA, CMS and ELWR, genotypes NARC-09, AUR-10, SA-75, Mexipak and AUR-09 are suitable for cultivation under drought conditions. The mentioned traits are encouraged to be used as screening criteria to breed for drought-tolerant wheat. This study recommends cell membrane stability (CMS) as better selection criterion in developing wheat lines for drought tolerance because it had positive correlation with Osmotic Adjustment (OA), Excised Leaf Water Retention (ELWR) and expressed negative correlation with canopy temperature (CT). Cooler canopy temperature with high CMS, OA, and ELWR indicates drought tolerance in bread wheat.
Acknowledgments
The corresponding author would like to thank the faculty and technical lab staff of cytogenetics, biotechnology, tissue culture and the central lab of Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Pakistan. The corrections and suggestions made by anonymous reviewers to improve this manuscript are highly appreciated.
Novelty Statement
Drought stress is a serious threat limiting global wheat (Triticum aestivum L.) production. However, little information is available in this regard. Various physiological parameters of wheat plant i.e. relative water content, excised leaf water retention, osmotic adjustment, canopy temperature etc. were studied to understand the effect of drought stress on its growth and development to identify drought tolerant gen-otypes based on physiological parameters.
Author’s Contribution
The concept, design and supervision of this research work were planned by MA. AD performed the experiment and analyzed the data. FMW provided technical assistance. AD and IA wrote the first draft of the manuscript. MKNS and SA made critical corrections in the manuscript.
References
Abd-El-Haleem S.H.M., M.A. Reham and S.M.S. Mohamed. 2009. Genetic analysis and RAPD polymorphism in some durum wheat genotypes. Gl. J. Biotech. Biochem. 4(1): 01-09.
Ahmadizadeh, M. 2013. Physiological and Agro-morphological response to drought stress. Middle-East J. Sci. Res. 13(8): 998-1009.
Akram, M. 2011. Growth and yield components of wheat under water stress of different growth stages. Bangladesh J. Agric. Res. 36(3): 455-468. https://doi.org/10.3329/bjar.v36i3.9264
Ali, M., A. Abbas, S.I. Awan, K. Jabran and S.D.A. Gardezi. 2011. The correlated response of various morpho-physiological characters with grain yield in sorghum landraces at different growth phases. J. Anim. Plant Sci. 21(4): 671-679.
Bates, L.S., R.P. Waldren and I.D. Teare. 1973. Rapid determination of free proline for water-stress studies. Plant Soil. 39(1): 205-207. https://doi.org/10.1007/BF00018060
Blum, A., N. Klueva and H. Nguyen. 2001. Wheat cellular thermotolerance is related to yield under heat stress. Euphytica. 117: 117–123. https://doi.org/10.1023/A:1004083305905
Cao, H.X., C.H. Sun, H.B. Shao and X.T. Lei. 2011. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr. J. Biotech. 10: 2630-2637. https://doi.org/10.5897/AJB10.1272
Chorfi, A. and K. Taibi. 2011. Biochemical screening for osmotic adjustment of wheat genotypes under drought stress. Tropicul. 29(2): 82-87.
Edgerton, M.D. 2009. Increasing crop productivity to meet global needs for feed, food and fuel. Plant Physiol. 149(1): 7-13. https://doi.org/10.1104/pp.108.130195
Egert, M. and M. Tevini. 2002. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot. 48(1): 43-49. https://doi.org/10.1016/S0098-8472(02)00008-4
Farooq, J., I. Khaliq, M.A. Ali, M. Khashif, A. Rehman, M. Naveed, Q. Ali, W. Nazeer and A. Farooq. 2011a. The inheritance pattern of yield attributes in spring wheat at grain filling stage under different temperature regimes. Aust. J. Crop Sci. 5(13): 1745-1753.
Farooq, J., I. Khaliq, M. Kashif, Q. Ali and S. Mapara. 2011b. Genetic analysis of relative cell injury percentage and some yield contributing traits in wheat under normal and heat stress conditions. Chill. J. Agric. Res. 71(4): 511-520. https://doi.org/10.4067/S0718-58392011000400003
Farshadfar, E., A. Afarinesh and J. Sutka. 2002. Inheritance of Drought Tolerance in Maize. CRC., 30: 3-4
Farshadfar, E., S. Jalali and M. Saeidi. 2012. Introduction of a new selection index for improvement of drought tolerance in common wheat (Triticum aestivum L.). Eur. J. Exp. Biol. 2(4): 1181-1187. https://doi.org/10.5539/jas.v4n9p1
Geravandi, M., E. Farshadfar and D. Kahrizi. 2011. Evaluation of some physiological traits as indicators of drought tolerance in bread wheat genotypes. Rus. J. Plant Physiol. 58(1): 69-75. https://doi.org/10.1134/S1021443711010067
Grover, A., A. Kapoor, D. Kumar, H.E. Shashidhar and S. Hittalmani. 2004. Genetic improvement for abiotic stress responses: In plant breeding mendelian to molecular approaches. Jain, HK., Kharkwal, M.C., Narosa, Publ. House, New Delhi, India. pp. 167-193. https://doi.org/10.1007/978-94-007-1040-5_8
Gunes, A., A. Inal, M.S. Adak, E.G. Bagci, N. Cicek and F. Eraslan. 2008. Effect of drought stress implemented at pre- or post-anthesis stage on some physiological parameters as screening criteria in chickpea cultivars. Russ. J. Plant Physiol. 55: 59-67. https://doi.org/10.1134/S102144370801007X
Hasheminasab, H., M.T. Assad, A. Aliakbari and S.R. Sahaffi. 2012. Evaluation of some physiological traits associated with improved drought tolerance in Iranian wheat. Ann. Boil. Res. 3(4): 1719-1725. https://doi.org/10.5539/jas.v4n8p20
Hennouni, N., M.R. Djebarand and H. Djebar-Berrebbah. 2012. Effect of systemic fungicide (the combination of Cyproconazole and propiconazole) newly introduced in Algeria on Septoria of two varieties of wheat (Triticum durum Desf). Advan. Environ. Biol. 6(4): 1433-1441.
Keshavars, L., H. Farahbaksh and P. Golkar. 2012. The effect of drought stress and super absorbent polymer on morph-physiological traits of pearl millet (Pennisetum glaucum). Int. Res. J. Appl. Basic Sci. 3(1): 148-154
Khaket, T.P., V. Kumar, J. Singh and S. Dhanda. 2014. Biochemical and physiological studies on the effects of senescence leaves of Populus deltoides on Triticum vulgare. Sci. World J. 2014. https://doi.org/10.1155/2014/126051
Khakwani, A.A., M.D. Dennett, M. Munir and M.S. Baloch. 2012. Wheat yield response to physiological limitations under water stress condition. J. Anim. Plant Sci. 22: 773-780.
Lugojan, C. and S. Ciulca. 2011. Evaluation of relative water content in winter wheat. J. Hort. For. Biotech. 15(2): 173-177.
Machado, S. and G. Paulsen. 2001. Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil. 233(2): 179-187. https://doi.org/10.1023/A:1010346601643
Mahpara, S. 2008. Biometrical analysis of important plant attributes in spring wheat, Ph. D. thesis, Dept. PBG. Univ. Agric. Faisalabad, Pakistan.
Maqbool, M.M., A. Ali, T. Haq, M.N. Majeed and D.J. Lee. 2015. The response of spring wheat (Triticum aestivum L.) to induced water stress at critical growth stages. Sarhad J. Agric. 31(1): 53-58.
Merah, O. 2001. The potential importance of water status traits for durum wheat improvement under Mediterranean conditions. J. Agric. Res. 137: 139-145. https://doi.org/10.1017/S0021859601001253
Ming, D.F., Z.F. Pei, M.S. Naeem, H.J. Gomg and W.J. Zhou. 2012. Silicon alleviates PEG-Induced Water-Deficit stress in upland Rice seedlings enhancing osmotic adjustment. J. Agron. Crop Sci. 198: 14-26. https://doi.org/10.1111/j.1439-037X.2011.00486.x
Moinuddin, R., A. Fischer, K.D. Sayre and M.P. Reynolds. 2005. Osmotic adjustment in wheat in relation to grain yield under water deficit environments. Agron. J. 97: 1062-1071. https://doi.org/10.2134/agronj2004.0152
Mujtaba, S.M. and S.M. Alam. 2002. Drought phenomenon and crop growth. Industry and economy. http://www.pakistaneconomist.com/issue2002/issue13/i&e4.htm. Pak. Gulf Econ., Pakistan. Accessed on 07.08.2018.
Naeem, M.K., M. Ahmad, M.K.N. Shah, M. Kamran and M.S. Iqbal. 2016. Character association and path analysis of osmotic adjustment, growth and physiological traits in wheat. J. Anim. Plant Sci. 26(3): 680-685.
Nezhadahmadi, A., Z.H. Prodhan and G. Faruq. 2013. Drought tolerance in wheat. Sci. W. J. 610-721. https://doi.org/10.1155/2013/610721
Nobel, P.S. 1991. Physicochemical and environmental plant physiology. In: Water. Academic Press, San Diego, Calif, USA. Chapter 2. pp. 54-55.
Nouri-Ganbalani, A., G. Nouri-Ganbalani and D. Hassanpanah. 2009. Effects of drought stress condition on the yield and yield components of advanced wheat genotypes in Ardabil, Iran. J. Food Agric. Environ. 7: 228-234.
Olivares-Villegas, J.J., M.P. Reynolds and G.K. McDonald. 2007. Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Funct. Plant Biol. 34: 189-203. https://doi.org/10.1071/FP06148
Premachandra, G.S. and T. Shimada. 1988. Evaluation of polyethylene glycol test of measuring cell membrane stability as a drought tolerance test in wheat. J. Agric. Sci. 110: 429-433. https://doi.org/10.1017/S002185960008196X
Rajaram, S. 2000. International wheat breeding: Past and present achievements and future directions. Oreg. State Univ. Ext. Ser. Spec. Rep. pp. 1017.
Razzaq, A., Q. Ali, A. Qayyum, I. Mahmood, M. Ahmad and M. Rasheed. 2013. Physiological responses and drought resistance index of nine wheat (Triticum aestivum L.) cultivars under different moisture conditions. Pak. J. Bot. 45(5): 151-155.
Rehman, S.U., M. Bilal, R. Rana, M. Tahir, M. Shah, H. Ayalew and G. Yan. 2016. Cell membrane stability and chlorophyll content variation in wheat (Triticum aestivum L) genotypes under heat and drought conditions. Crop Pasture Sci. 67: 712-718. https://doi.org/10.1071/CP15385
Sairam, R.K., K.V. Rao and G.C. Srivastava. 2002. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci., 163: 1037-1046. https://doi.org/10.1016/S0168-9452(02)00278-9
SAS Institute Inc. 2009. SAS/STAT ® 9.2 User’s Guide, Second Edition. Copyright © 2009, SAS Inst. Inc., Cary, NC, USA. (https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm)
Sayar, R., H. Khemira, A. Kameli and M. Mosbahi. 2008. Physiological tests as predictive appreciation for drought tolerance in durum wheat (Triticum durum Desf.). Agron. Res. 6(1): 79-90.
Shamsi, K. 2010. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 8: 1051-1060.
Sharafi, S., G.K. Ghassemi, S. Mohammadi, S. Lak and B. Sorkhy. 2011. Evaluation of drought tolerance and yield potential in winter barley (Hordeum vulgare) varieties. JFAE. 9: 419-422.
Steel, R.G.D., J.H. Torrie and D.A. Deekey. 1997. Principles and procedures of statistics: A biometrical approach. 3rd edn. McGraw Hill, New York. pp. 633.
Sultan, M.A.R.F., L. Hui, L.J. Yang and Z.H. Xian. 2012. Assessment of Drought Tolerance of Some Triticum L. Species through Physiological Indices. Czech J. Genet. Plant Breed. 48(4): 178-18. https://doi.org/10.17221/21/2012-CJGPB
Zaharieva, M., E. Gaulin, M. Havaux, E. Acevedo and P. Monnevaux. 2001. Drought and heat responses in the wild wheat relative Aegilops Geniculata Roth. Crop Sci. 41: 1321-1329. https://doi.org/10.2135/cropsci2001.4141321x
To share on other social networks, click on any share button. What are these?








